
Contributions
Abstract: PB2373
Type: Publication Only
Background
Sickle Cell Disease (SCD) is a chronic and complex multisystem disorder requiring comprehensive care with newborn screening (NBS), health education, management of acute and chronic complications. Early diagnosis through NBS programs allows timely implementation of preventive measures such as penicillin prophylaxis, adequate health care measures (i.e spleen palpation), stroke prevention programs and hydroxyurea treatment. In spite of evidence of the above benefits of NBS for SCD and the inclusion of NBS as first step for comprehensive care in international guidelines, Italy still lacks a national SCD NBS program and policy on blood disorders. Pilot single center screening programs and a regional targeted screening have been implemented so far, but more evidence is needed to impact health policies
Aims
To evaluate feasibility and efficacy of a multi center universal newborn screening for SCD in the frame work of a public-private partnership; to determine SCD epidemiology in the areas of Padova and Monza (North Italy) as rationale to support the need for a national universal SCD NBS
Methods
Two public tertiary care university hospitals, Reference Centers for Pediatric Hematologic Disorders- including SCD, partnered with local charities to organize the NBS program. Guthrie cards for HPLC analysis were collected for newborns in both centers' nursery and NICU, after informed consent from the mothers. The analysis were done in the Padova lab. Confirmation test with molecular genetics was performed for HPLC positive samples. SCD patients were enrolled in comprehensive care programs; S carriers were offered genetic counseling extended to the family
Results
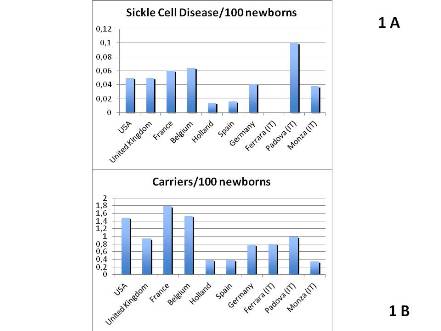
5466 newborns were enrolled and for 5439 informed consents were obtained. All the samples were adequate for analysis. A similar families origin was seen in the two centers (65% Italians, 9% Mixed Couples, 26% Immigrants), but Padova had higher percentage of immigrants (>60%) coming from areas at high risk of hemoglobinopathies. The incidence of SCD patients, traits and other Hb abnormalities are showed in Table 1. Compared to other SCD NBS programs in USA and Europe, our results show similar incidence of SCD patients and carriers (Figures 1 A and B). SCD patients were all Sub-Saharian Africans, while HbS and other variants carriers were: 15% and 23% Caucasians (Italian and Albanians); 10% and 47% from North Africa-India-South America respectively
Table 1. The incidence of SCD patients, traits and other Hb abnormalities
| Newborn | Positive test | Newborn SCD | Newborn S carriers | Other hemoglobinophathies |
Padova (PD) | 2821 | 37 (1.27%) | 3 (0.10%) | 28 (0.99%) | 6 (0.21%) |
Monza (MZ) | 2618 | 23 (0.88%) | 1 (0.038%) | 9 (0.34%) | 13 (0.5%) |
MZ + PD | 5439 | 60 (1.1%) | 4 (0.07%) | 37 (0.68%) | 19 (0.34%) |
Conclusion
Our results demonstrate the feasibility of a multicentric NBS program for SCD; give information on HbS epidemiology in two Northern Italian Areas and support previous European recommendation for a universal NBS program for SCD in Italy: a high incidence of patients and carriers was detected, with high percentage of carriers of non Sub-Saharian African origin, impossible to identify in a targeted NBS which is therefore not not adequate in our context.
Session topic: 36. Quality of life, palliative care, ethics and health economics
Keyword(s): Childhood, Health care, Screening, sickle cell disease
Abstract: PB2373
Type: Publication Only
Background
Sickle Cell Disease (SCD) is a chronic and complex multisystem disorder requiring comprehensive care with newborn screening (NBS), health education, management of acute and chronic complications. Early diagnosis through NBS programs allows timely implementation of preventive measures such as penicillin prophylaxis, adequate health care measures (i.e spleen palpation), stroke prevention programs and hydroxyurea treatment. In spite of evidence of the above benefits of NBS for SCD and the inclusion of NBS as first step for comprehensive care in international guidelines, Italy still lacks a national SCD NBS program and policy on blood disorders. Pilot single center screening programs and a regional targeted screening have been implemented so far, but more evidence is needed to impact health policies
Aims
To evaluate feasibility and efficacy of a multi center universal newborn screening for SCD in the frame work of a public-private partnership; to determine SCD epidemiology in the areas of Padova and Monza (North Italy) as rationale to support the need for a national universal SCD NBS
Methods
Two public tertiary care university hospitals, Reference Centers for Pediatric Hematologic Disorders- including SCD, partnered with local charities to organize the NBS program. Guthrie cards for HPLC analysis were collected for newborns in both centers' nursery and NICU, after informed consent from the mothers. The analysis were done in the Padova lab. Confirmation test with molecular genetics was performed for HPLC positive samples. SCD patients were enrolled in comprehensive care programs; S carriers were offered genetic counseling extended to the family
Results
5466 newborns were enrolled and for 5439 informed consents were obtained. All the samples were adequate for analysis. A similar families origin was seen in the two centers (65% Italians, 9% Mixed Couples, 26% Immigrants), but Padova had higher percentage of immigrants (>60%) coming from areas at high risk of hemoglobinopathies. The incidence of SCD patients, traits and other Hb abnormalities are showed in Table 1. Compared to other SCD NBS programs in USA and Europe, our results show similar incidence of SCD patients and carriers (Figures 1 A and B). SCD patients were all Sub-Saharian Africans, while HbS and other variants carriers were: 15% and 23% Caucasians (Italian and Albanians); 10% and 47% from North Africa-India-South America respectively
Table 1. The incidence of SCD patients, traits and other Hb abnormalities
| Newborn | Positive test | Newborn SCD | Newborn S carriers | Other hemoglobinophathies |
Padova (PD) | 2821 | 37 (1.27%) | 3 (0.10%) | 28 (0.99%) | 6 (0.21%) |
Monza (MZ) | 2618 | 23 (0.88%) | 1 (0.038%) | 9 (0.34%) | 13 (0.5%) |
MZ + PD | 5439 | 60 (1.1%) | 4 (0.07%) | 37 (0.68%) | 19 (0.34%) |

Conclusion
Our results demonstrate the feasibility of a multicentric NBS program for SCD; give information on HbS epidemiology in two Northern Italian Areas and support previous European recommendation for a universal NBS program for SCD in Italy: a high incidence of patients and carriers was detected, with high percentage of carriers of non Sub-Saharian African origin, impossible to identify in a targeted NBS which is therefore not not adequate in our context.
Session topic: 36. Quality of life, palliative care, ethics and health economics
Keyword(s): Childhood, Health care, Screening, sickle cell disease


