
Contributions
Abstract: PB1770
Type: Publication Only
Background
R-CHOP remains the standard of care in patients with previously untreated diffuse large B-cell lymphoma (DLBCL). However, patients with high-risk disease have poorer outcomes with R-CHOP. Polatuzumab vedotin (pola) is an antibody-drug conjugate targeting CD79b; it delivers the antimitotic agent MMAE and is being evaluated as a replacement strategy for vincristine within the R-CHOP regimen. In a phase Ib/II study in higher risk DLBCL patients, pola + R-CHP produced promising efficacy across different subtypes of DLBCL and a safety profile similar to that observed in the R-CHOP arm of the GOYA study (Tilly H, et al. Hematol Oncol 2017; Vitolo U, et al. J Clin Oncol 2017).
Aims
To evaluate the efficacy and safety of pola plus R-CHP compared with R-CHOP in patients with previously untreated DLBCL to see whether pola can improve outcomes in patients with low to high-risk disease.
Methods
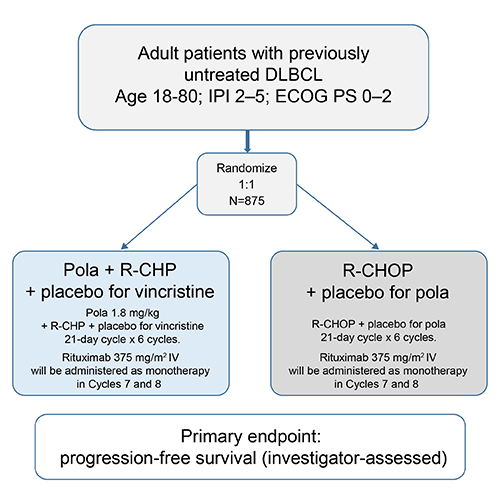
This is a multicenter, randomized, double-blind, placebo-controlled, phase 3 study in patients with previously untreated DLBCL. Patients (planned N = 875) aged 18-80 years with CD20-positive DLBCL (including DLBCL not otherwise specified [NOS], germinal center B-cell like [GCB], and activated B-cell like [ABC] subtypes), ECOG performance status 0–2, and IPI score 2–5, will be randomized 1:1 to one of two treatment groups, stratified by IPI score (2 versus 3–5), bulky disease and geographical region (Figure). Arm A will receive pola 1.8 mg/kg on Day 1 plus R-CHP (standard dosing schedule) plus vincristine placebo for 6 cycles; Arm B will receive R-CHOP (standard dosing schedule) with pola placebo for 6 cycles. In both arms, R will be administered as monotherapy in cycles 7 and 8.
The primary endpoint is progression-free survival (PFS), as assessed by the investigator, using the Lugano classification (Cheson B, et al. J Clin Oncol 2014). Secondary endpoints include PET-CT complete response rate at end of treatment assessed by an independent review committee, event-free survival due to efficacy reason, 2-year PFS rate, and overall survival.
PET-CT and CT scans will be obtained at screening, after 4 cycles (planned interim assessment), and 6–8 weeks after end of study treatment. Patient follow-up will continue for 5 years after end of treatment.
Results
Enrolment began November 2017.
Conclusion
This phase 3 study, POLARIX, will evaluate clinical outcomes with pola plus R-CHP compared with R-CHOP in patients with previously untreated DLBCL. Clinical trial information: the study is funded by F. Hoffmann-La Roche Ltd; NCT03274492.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Diffuse large B cell lymphoma, Immunoconjugate, Phase III
Abstract: PB1770
Type: Publication Only
Background
R-CHOP remains the standard of care in patients with previously untreated diffuse large B-cell lymphoma (DLBCL). However, patients with high-risk disease have poorer outcomes with R-CHOP. Polatuzumab vedotin (pola) is an antibody-drug conjugate targeting CD79b; it delivers the antimitotic agent MMAE and is being evaluated as a replacement strategy for vincristine within the R-CHOP regimen. In a phase Ib/II study in higher risk DLBCL patients, pola + R-CHP produced promising efficacy across different subtypes of DLBCL and a safety profile similar to that observed in the R-CHOP arm of the GOYA study (Tilly H, et al. Hematol Oncol 2017; Vitolo U, et al. J Clin Oncol 2017).
Aims
To evaluate the efficacy and safety of pola plus R-CHP compared with R-CHOP in patients with previously untreated DLBCL to see whether pola can improve outcomes in patients with low to high-risk disease.
Methods
This is a multicenter, randomized, double-blind, placebo-controlled, phase 3 study in patients with previously untreated DLBCL. Patients (planned N = 875) aged 18-80 years with CD20-positive DLBCL (including DLBCL not otherwise specified [NOS], germinal center B-cell like [GCB], and activated B-cell like [ABC] subtypes), ECOG performance status 0–2, and IPI score 2–5, will be randomized 1:1 to one of two treatment groups, stratified by IPI score (2 versus 3–5), bulky disease and geographical region (Figure). Arm A will receive pola 1.8 mg/kg on Day 1 plus R-CHP (standard dosing schedule) plus vincristine placebo for 6 cycles; Arm B will receive R-CHOP (standard dosing schedule) with pola placebo for 6 cycles. In both arms, R will be administered as monotherapy in cycles 7 and 8.
The primary endpoint is progression-free survival (PFS), as assessed by the investigator, using the Lugano classification (Cheson B, et al. J Clin Oncol 2014). Secondary endpoints include PET-CT complete response rate at end of treatment assessed by an independent review committee, event-free survival due to efficacy reason, 2-year PFS rate, and overall survival.
PET-CT and CT scans will be obtained at screening, after 4 cycles (planned interim assessment), and 6–8 weeks after end of study treatment. Patient follow-up will continue for 5 years after end of treatment.
Results
Enrolment began November 2017.

Conclusion
This phase 3 study, POLARIX, will evaluate clinical outcomes with pola plus R-CHP compared with R-CHOP in patients with previously untreated DLBCL. Clinical trial information: the study is funded by F. Hoffmann-La Roche Ltd; NCT03274492.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Diffuse large B cell lymphoma, Immunoconjugate, Phase III


