
Contributions
Abstract: PB1631
Type: Publication Only
Background
Relapsed ALL in adult carries a dismal prognosis despite intensive treatment.
Aims
In this report, successful alternative treatment containing rituximab and infusion of Dendritic cells-CIK is described in a case of early relapsed B cell ALL patient who could not tolerate intensive chemotherapy.
Methods
A 52-year-old man first presented with persisting fever and weakness in May. 2014. A blood test showed decreased levels of white blood cell(1.8*10^9/L) and platelets (63*10^9/L). Bone marrow aspirate revealed 72 percent primitive blast cells, and immunophenotypic analysis showed that the blast cells expressed CD10, CD19, CD22, CD79a and human leukocyte antigen D–related. No cytogenetic abnormalities were detected by G-banding analysis. Similarly, MLL gene rearrangement, BCR/ABL1, ETV5/RUNX1 were also negative. Diagnosis of B-lymphoblastic leukemia NOS was made on basis of 2016 update revision of WHO classification criteria. The patient received VDCLP regimen consisting of vindesine, idarubicin, cyclophosphamide, asparaginase and dexamethasone. Finally, he achieved complete remission (CR) with 3.5% blast cells in bone marrow. However, severe side effects induced by induction therapy including febrile neutropenia, hyperglycemia and hepatic toxicity were followed. After high-dose methotrexate combined with asparaginase and MA consolidation therapy, neutropenia lasted for 2 months, during which he suffered pulmonary infection, sepsis and liver dysfunction. He refused to receive any maintenance therapy for significant complications.
Results
Six months later, the patient presented with fatigue and petechiae with a short CR1. Bone marrow with 31% blast cells was consistent with relapsed B cell ALL (CD19+, CD20+, CD10+, CD22+) (Fig). Treatment approaches for this patient in poor performance status is limited. DC-CIK was chosen as therapeutic choice. Subsequently, rituximab combined VDLP regimen was adopted, and he got second remission. Thereafter, four times consolidation therapy including rituximab were processed, during which seven cycles of DC-CIK cells infusion (each (4–6) × 109 CIK cells) were also accomplished. After that he received rituximab alone every 3 months for a year, and took thioguanine and methotrexate tablets at intervals of immunotherapy until March 11, 2017. Impressively, the patient has been in bone marrow remission for 30 months since last relapse.
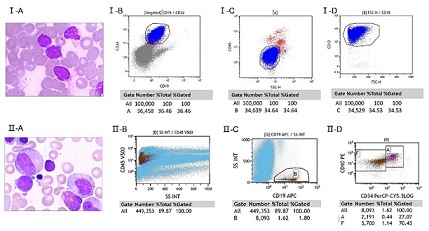
Conclusion
High reinduction mortality, chemotherapy resistance and disease recurrent are impediments to successful management in relapsed ALL patients. The advantage of DC-CIK therapy is no adverse reactions, because CIK cells’ anti-tumor activity is perforin mediated and various receptors were involved. Recent studies demonstrated that rituximab, anti-CD20 monoclonal antibody, may play a critical role in improvement of CIK-mediated cytotoxicity to leukemia cell. In this case, initiating DC-CIK therapy before chemotherapy help to improve the tolerability in patients with significant complications. To the best of our knowledge, it’s the first report on DC-CIK combined with rituximab-based regimen for recurrent ALL.AcknowledgmentThe research was supported by funding of the Science and Technology Department of Zhejiang Province, China(2016C33160), and Yiwu public technology research projects, Zhejiang Province, China (2016-S-05).Correspondence to: Dr Jian Huang, .E-mail: househuang@zju.edu.cn
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Cytokine, Dendritic cell, Relapsed acute lymphoblastic leukemia, Treatment
Abstract: PB1631
Type: Publication Only
Background
Relapsed ALL in adult carries a dismal prognosis despite intensive treatment.
Aims
In this report, successful alternative treatment containing rituximab and infusion of Dendritic cells-CIK is described in a case of early relapsed B cell ALL patient who could not tolerate intensive chemotherapy.
Methods
A 52-year-old man first presented with persisting fever and weakness in May. 2014. A blood test showed decreased levels of white blood cell(1.8*10^9/L) and platelets (63*10^9/L). Bone marrow aspirate revealed 72 percent primitive blast cells, and immunophenotypic analysis showed that the blast cells expressed CD10, CD19, CD22, CD79a and human leukocyte antigen D–related. No cytogenetic abnormalities were detected by G-banding analysis. Similarly, MLL gene rearrangement, BCR/ABL1, ETV5/RUNX1 were also negative. Diagnosis of B-lymphoblastic leukemia NOS was made on basis of 2016 update revision of WHO classification criteria. The patient received VDCLP regimen consisting of vindesine, idarubicin, cyclophosphamide, asparaginase and dexamethasone. Finally, he achieved complete remission (CR) with 3.5% blast cells in bone marrow. However, severe side effects induced by induction therapy including febrile neutropenia, hyperglycemia and hepatic toxicity were followed. After high-dose methotrexate combined with asparaginase and MA consolidation therapy, neutropenia lasted for 2 months, during which he suffered pulmonary infection, sepsis and liver dysfunction. He refused to receive any maintenance therapy for significant complications.
Results
Six months later, the patient presented with fatigue and petechiae with a short CR1. Bone marrow with 31% blast cells was consistent with relapsed B cell ALL (CD19+, CD20+, CD10+, CD22+) (Fig). Treatment approaches for this patient in poor performance status is limited. DC-CIK was chosen as therapeutic choice. Subsequently, rituximab combined VDLP regimen was adopted, and he got second remission. Thereafter, four times consolidation therapy including rituximab were processed, during which seven cycles of DC-CIK cells infusion (each (4–6) × 109 CIK cells) were also accomplished. After that he received rituximab alone every 3 months for a year, and took thioguanine and methotrexate tablets at intervals of immunotherapy until March 11, 2017. Impressively, the patient has been in bone marrow remission for 30 months since last relapse.

Conclusion
High reinduction mortality, chemotherapy resistance and disease recurrent are impediments to successful management in relapsed ALL patients. The advantage of DC-CIK therapy is no adverse reactions, because CIK cells’ anti-tumor activity is perforin mediated and various receptors were involved. Recent studies demonstrated that rituximab, anti-CD20 monoclonal antibody, may play a critical role in improvement of CIK-mediated cytotoxicity to leukemia cell. In this case, initiating DC-CIK therapy before chemotherapy help to improve the tolerability in patients with significant complications. To the best of our knowledge, it’s the first report on DC-CIK combined with rituximab-based regimen for recurrent ALL.AcknowledgmentThe research was supported by funding of the Science and Technology Department of Zhejiang Province, China(2016C33160), and Yiwu public technology research projects, Zhejiang Province, China (2016-S-05).Correspondence to: Dr Jian Huang, .E-mail: househuang@zju.edu.cn
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Cytokine, Dendritic cell, Relapsed acute lymphoblastic leukemia, Treatment


