
Contributions
Abstract: PB2153
Type: Publication Only
Background
Carfilzomib (CFZ) is a next-generation proteasome inhibitor currently approved in combination with lenalidomide and dexamethasone (KRd) or with dexamethasone alone (Kd) for treatment (Tx) of adult multiple myeloma (MM) patients (pts) with at least one prior Tx in the European Union (EU). Access to CFZ is ongoing throughout the EU. Real-world evidence is crucial to understand how CFZ based regimens are used in practice and in relation to EU prescribing information (PI).
Aims
To describe CFZ utilisation in routine clinical practice as well as the pt population, safety profile, response to Tx and selected healthcare resource utilisation. Results from a first planned interim analysis are reported.
Methods
This prospective cohort study (NCT03091127) recruited adults who at the time of CFZ initiation had experienced a relapse, received ≥1 prior line of MM Tx and ≥1 dose of CFZ in a combination regimen in routine clinical practice. Medical history and pt characteristics prior to CFZ initiation are collected as well as further data during pt observation until 30 days after final CFZ administration or until 18 months after initiation, whichever is earlier. All adverse events of grade 3 or above (Gr3+, including serious adverse events [SAE]) are collected.
Results
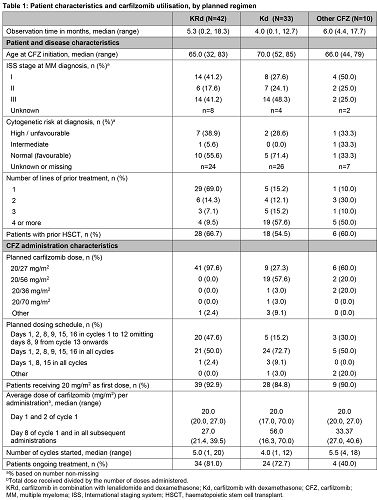
First pt was enrolled on 14th March 2017. As of 30 October 2017, 85 pts have been included from 4 countries participating so far: Austria (22), Belgium (23), Greece (26), and the Netherlands (14). The reported regimens planned to be prescribed were KRd (49%), Kd (39%), and other CFZ regimen combinations (12%) which included triplets with cyclophosphamide (n=5), pomalidomide (n=3), methylprednisolone (n=1), and elotuzumab (n=1). Overall, 34.1% of pts reported a history of hypertension, 15.3% of cardiac disorders, 15.3% of diabetes and 5.9% of pulmonary embolism prior to CFZ. On average, pts with planned KRd were younger than pts with planned Kd and 69% of KRd pts had received 1 prior line of Tx compared with 15% of Kd pts. For nearly all KRd pts (97.6%) and over half of Kd pts (57.6%), the planned CFZ dose was per EU PI, 20/27 mg/m2 and 20/56 mg/m2 respectively. For all remaining Kd pts physicians planned a dose lower than the recommended biweekly dose of 112 mg/m2. For nearly half of KRd pts (47.6%) and around three quarters of Kd pts (72.7%), the planned administration schedule was per EU PI. Regardless of regimen, most pts received the starting dose, 20 mg/m2. The average dose for administrations from day 8 of cycle 1 onwards was largely in line with the EU PI. After a median observation time of 4.6 months, 81% and 73% of KRd and Kd pts, respectively, are still ongoing CFZ Tx. Treatment-emergent adverse events Gr3+ were reported in 26.2% of KRd (16.7% SAEs), 21.2% of Kd (18.2% SAEs) and 60% of other combinations (10.0% SAEs) and one fatal event of cardio-respiratory arrest occurred in the Kd group. The most frequent events were neutropenia (n=4), pneumonia (n=4), anaemia (n=3), decreased platelet count (n=3) and sepsis (n=2) and one pt experienced cardiac failure. Only 3 pts discontinued CFZ Tx due to an AE.
Conclusion
These first results suggest that KRd may be preferably used as second-line Tx and Kd may be deferred to later Tx lines, however, KRd was generally available before Kd in participating countries. The planned CFZ dose reduction among Kd pts may reflect a real-life approach to manage frail and heavily pretreated pts. Further observation of increasing number of pts in routine practice with further follow up is ongoing to assess longer-term pt management and response.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): epidemiology, Multiple Myeloma, Proteasome inhibitor, Treatment
Abstract: PB2153
Type: Publication Only
Background
Carfilzomib (CFZ) is a next-generation proteasome inhibitor currently approved in combination with lenalidomide and dexamethasone (KRd) or with dexamethasone alone (Kd) for treatment (Tx) of adult multiple myeloma (MM) patients (pts) with at least one prior Tx in the European Union (EU). Access to CFZ is ongoing throughout the EU. Real-world evidence is crucial to understand how CFZ based regimens are used in practice and in relation to EU prescribing information (PI).
Aims
To describe CFZ utilisation in routine clinical practice as well as the pt population, safety profile, response to Tx and selected healthcare resource utilisation. Results from a first planned interim analysis are reported.
Methods
This prospective cohort study (NCT03091127) recruited adults who at the time of CFZ initiation had experienced a relapse, received ≥1 prior line of MM Tx and ≥1 dose of CFZ in a combination regimen in routine clinical practice. Medical history and pt characteristics prior to CFZ initiation are collected as well as further data during pt observation until 30 days after final CFZ administration or until 18 months after initiation, whichever is earlier. All adverse events of grade 3 or above (Gr3+, including serious adverse events [SAE]) are collected.
Results
First pt was enrolled on 14th March 2017. As of 30 October 2017, 85 pts have been included from 4 countries participating so far: Austria (22), Belgium (23), Greece (26), and the Netherlands (14). The reported regimens planned to be prescribed were KRd (49%), Kd (39%), and other CFZ regimen combinations (12%) which included triplets with cyclophosphamide (n=5), pomalidomide (n=3), methylprednisolone (n=1), and elotuzumab (n=1). Overall, 34.1% of pts reported a history of hypertension, 15.3% of cardiac disorders, 15.3% of diabetes and 5.9% of pulmonary embolism prior to CFZ. On average, pts with planned KRd were younger than pts with planned Kd and 69% of KRd pts had received 1 prior line of Tx compared with 15% of Kd pts. For nearly all KRd pts (97.6%) and over half of Kd pts (57.6%), the planned CFZ dose was per EU PI, 20/27 mg/m2 and 20/56 mg/m2 respectively. For all remaining Kd pts physicians planned a dose lower than the recommended biweekly dose of 112 mg/m2. For nearly half of KRd pts (47.6%) and around three quarters of Kd pts (72.7%), the planned administration schedule was per EU PI. Regardless of regimen, most pts received the starting dose, 20 mg/m2. The average dose for administrations from day 8 of cycle 1 onwards was largely in line with the EU PI. After a median observation time of 4.6 months, 81% and 73% of KRd and Kd pts, respectively, are still ongoing CFZ Tx. Treatment-emergent adverse events Gr3+ were reported in 26.2% of KRd (16.7% SAEs), 21.2% of Kd (18.2% SAEs) and 60% of other combinations (10.0% SAEs) and one fatal event of cardio-respiratory arrest occurred in the Kd group. The most frequent events were neutropenia (n=4), pneumonia (n=4), anaemia (n=3), decreased platelet count (n=3) and sepsis (n=2) and one pt experienced cardiac failure. Only 3 pts discontinued CFZ Tx due to an AE.

Conclusion
These first results suggest that KRd may be preferably used as second-line Tx and Kd may be deferred to later Tx lines, however, KRd was generally available before Kd in participating countries. The planned CFZ dose reduction among Kd pts may reflect a real-life approach to manage frail and heavily pretreated pts. Further observation of increasing number of pts in routine practice with further follow up is ongoing to assess longer-term pt management and response.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): epidemiology, Multiple Myeloma, Proteasome inhibitor, Treatment


