
Contributions
Abstract: S1554
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 09:00 - 09:15
Location: Room A2
Background
MAJIC-ET a phase 2 study evaluated the role of ruxolitinib in hydroxycarbamide resistant/intolerant (HC-RES/INT) essential thrombocythaemia (ET). The impact of mutations in addition to recurrent driver mutations (JAK2/CALR/MPL) in RES/INT ET is currently unknown.
Aims
Here we expand the primary MAJIC-ET analysis to evaluate non-driver mutational profiles of MAJIC-ET patients (pts) using an ISO accredited Illumina TruSeq Custom Amplicon Panel, including 31 gene mutation hotspots & exons (~36,000 bp, 287 amplicons), followed by correlation with clinical outcomes.
Methods
Data was censored in January 2017 with 47.3% (n=52) randomised to best available therapy (BAT) & 52.7% (n=58) to ruxolitinib. Baseline clinical parameters including driver mutation status were matched.
Results
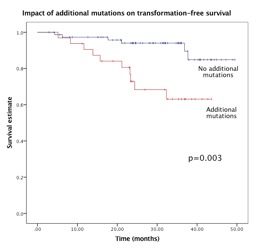
Overall, JAK2, CALR & MPL mutations were present in 48.6%, 30.3% & 4.6% of pts with 16.5% reported as “triple-negative”. At one-year, responses were equivalent in BAT & ruxolitinib; 44.2% vs 46.6%, respectively, p=0.4. Molecular responses were rare, with complete molecular response (CMR) only in two CALR- & JAK2-mutated ruxolitinib & not BAT-treated pts. Additional mutations at baseline were detected in 29.1%. One additional mutation was present in 20% (n=22) & ≥2 mutations in 9.1% (n=10). The most frequently identified mutation was TET2 (n=14) followed by TP53 (n=8) & SF3B1 (n=7). The latter two detected at higher frequencies than previously reported in ET. TP53 mutations were more frequent in “triple-negative” (16.7%) than in JAK2/CALR/MPL-mutated pts (4.4%), p=0.087. Presence of additional mutations was not predictive of responses. Only one pt with a molecular response (partial) had a baseline additional mutation; JAK2 V617F co-mutated with TP53. Notably, one CALR-mutated pt with a CMR subsequently transformed to post-ET myelofibrosis with no additional mutation at baseline; mutational analysis at transformation is underway. Overall survival (OS) at 2 years was 95.7% in BAT & 93.6% in ruxolitinib-treated pts, p=0.264. OS was not affected by driver mutation status/allele burden or additional mutations. Transformation-free survival (TFS) at 2 years was similar in BAT (88.7%, 95% CI 77–94%) & ruxolitinib-treated pts (81%, 95% CI 70–88%), p=0.078. Presence of non-driver mutations was associated with increased risk of transformation (OR 8.1, 95% CI 1.9-33), p=0.003 & predictive of an inferior 2-year TFS of 67.2% (95% CI 54–77%) vs. 94.2% (95% CI 86–97%) in pts with no additional mutations, p=0.003 (Figure 1). This remained significant on subgroup analysis of BAT-treated (p=0.016) but not for the ruxolitinib-treated pts.Presence of a TP53 mutation was associated with a poorer 2-year TFS: 54% (95% CI 31–72%) in TP53-mutated vs. 88% (95% CI 80-92%) in TP53 non-mutated pts, p=0.042. TP53 mutations were not associated with prior busulfan/32P/Pipobroman. Presence of an SF3B1 mutation predicted a 2-year TFS of 57.1 (95% CI 34–74%) in SF3B1-mutated vs. 89% (95% CI 82–93%) in SF3B1 non-mutated pts, p=0.001. High JAK2 allele burden (≥50%) did not predict transformation risk & neither JAK2 allele burden nor presence of additional mutations increased the risk of thrombotic events.
Conclusion
We report for the first time a comprehensive mutational analysis of HC-RES/INT ET pts within the context of a clinical trial demonstrating a distinct mutational profile in this cohort. The presence of additional mutations was predictive for adverse TFS & we observed a high prevalence of TP53 & SF3B1 mutations. Our data highlight the clinical/prognostic utility of more extensive mutation screening in HC RES/INT ET.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Essential Thrombocytemia, mutation analysis, Myeloproliferative disorder, Ruxolitinib
Abstract: S1554
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 09:00 - 09:15
Location: Room A2
Background
MAJIC-ET a phase 2 study evaluated the role of ruxolitinib in hydroxycarbamide resistant/intolerant (HC-RES/INT) essential thrombocythaemia (ET). The impact of mutations in addition to recurrent driver mutations (JAK2/CALR/MPL) in RES/INT ET is currently unknown.
Aims
Here we expand the primary MAJIC-ET analysis to evaluate non-driver mutational profiles of MAJIC-ET patients (pts) using an ISO accredited Illumina TruSeq Custom Amplicon Panel, including 31 gene mutation hotspots & exons (~36,000 bp, 287 amplicons), followed by correlation with clinical outcomes.
Methods
Data was censored in January 2017 with 47.3% (n=52) randomised to best available therapy (BAT) & 52.7% (n=58) to ruxolitinib. Baseline clinical parameters including driver mutation status were matched.
Results
Overall, JAK2, CALR & MPL mutations were present in 48.6%, 30.3% & 4.6% of pts with 16.5% reported as “triple-negative”. At one-year, responses were equivalent in BAT & ruxolitinib; 44.2% vs 46.6%, respectively, p=0.4. Molecular responses were rare, with complete molecular response (CMR) only in two CALR- & JAK2-mutated ruxolitinib & not BAT-treated pts. Additional mutations at baseline were detected in 29.1%. One additional mutation was present in 20% (n=22) & ≥2 mutations in 9.1% (n=10). The most frequently identified mutation was TET2 (n=14) followed by TP53 (n=8) & SF3B1 (n=7). The latter two detected at higher frequencies than previously reported in ET. TP53 mutations were more frequent in “triple-negative” (16.7%) than in JAK2/CALR/MPL-mutated pts (4.4%), p=0.087. Presence of additional mutations was not predictive of responses. Only one pt with a molecular response (partial) had a baseline additional mutation; JAK2 V617F co-mutated with TP53. Notably, one CALR-mutated pt with a CMR subsequently transformed to post-ET myelofibrosis with no additional mutation at baseline; mutational analysis at transformation is underway. Overall survival (OS) at 2 years was 95.7% in BAT & 93.6% in ruxolitinib-treated pts, p=0.264. OS was not affected by driver mutation status/allele burden or additional mutations. Transformation-free survival (TFS) at 2 years was similar in BAT (88.7%, 95% CI 77–94%) & ruxolitinib-treated pts (81%, 95% CI 70–88%), p=0.078. Presence of non-driver mutations was associated with increased risk of transformation (OR 8.1, 95% CI 1.9-33), p=0.003 & predictive of an inferior 2-year TFS of 67.2% (95% CI 54–77%) vs. 94.2% (95% CI 86–97%) in pts with no additional mutations, p=0.003 (Figure 1). This remained significant on subgroup analysis of BAT-treated (p=0.016) but not for the ruxolitinib-treated pts.Presence of a TP53 mutation was associated with a poorer 2-year TFS: 54% (95% CI 31–72%) in TP53-mutated vs. 88% (95% CI 80-92%) in TP53 non-mutated pts, p=0.042. TP53 mutations were not associated with prior busulfan/32P/Pipobroman. Presence of an SF3B1 mutation predicted a 2-year TFS of 57.1 (95% CI 34–74%) in SF3B1-mutated vs. 89% (95% CI 82–93%) in SF3B1 non-mutated pts, p=0.001. High JAK2 allele burden (≥50%) did not predict transformation risk & neither JAK2 allele burden nor presence of additional mutations increased the risk of thrombotic events.

Conclusion
We report for the first time a comprehensive mutational analysis of HC-RES/INT ET pts within the context of a clinical trial demonstrating a distinct mutational profile in this cohort. The presence of additional mutations was predictive for adverse TFS & we observed a high prevalence of TP53 & SF3B1 mutations. Our data highlight the clinical/prognostic utility of more extensive mutation screening in HC RES/INT ET.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Essential Thrombocytemia, mutation analysis, Myeloproliferative disorder, Ruxolitinib


