
Contributions
Abstract: S1549
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 09:00 - 09:15
Location: Room A1
Background
The Italian phase III randomized study FIL-DLCL04 (Chiappella et al, Lancet Oncol 2017) showed that an abbreviated Rituximab-CHOP dose-dense chemotherapy followed by consolidation with Rituximab-high dose chemotherapy and autologous stem cell transplantation (R-HDC+ASCT) compared to a full course of R-CHOP dose-dense chemotherapy without consolidation, determined an advantage in term of failure-free survival (FFS) but no difference in overall survival (OS) in young patients with untreated diffuse-large B-cell lymphomas (DLBCL) at high-risk. The prognostic role of TP53 is well-known in chronic lymphocytic leukaemia, but it has not yet been established in DLBCL.
Aims
Aim of this analysis was to correlate TP53 mutations, cell of origin (COO) profile and the presence of biomarkers (MYC, BCL2), with OS and FFS.
Methods
From 2005 to 2010, 399 young untreated DLBCL at poor-risk, were enrolled in FIL-DLCL04 and randomized to receive R-HDC+ASCT in 199 and R-dose-dense in 200 (NCT00499018). TP53 disruption by gene mutation was analyzed by Sanger DNA sequencing. COO classification as germinal center (GCB), activated B-cell (ABC) and unclassified was based on gene-expression profiling using the NanoString research use only lymphoma subtyping test. BCL2, MYC and TP53 were studied in immunohistochemistry (IHC); cases were deemed positive if at least 50%, 40% and 30% of lymphoma cells were stained with BCL2, c-MYC and TP53 antibodies, respectively. Bcl2 and Myc translocations, copy gains and aberrations were tested by fluorescent in situ hybridization (FISH). OS and FFS were analyzed; a crude hazard ratio (HR) and an adjusted HR (aHR) for clinical characteristics (age, treatment, gender, age-adjusted International Prognostic Index, performance status, bone marrow involvement) and for biological factors (double-expressors, double-hit, COO profile) were calculated.
Results
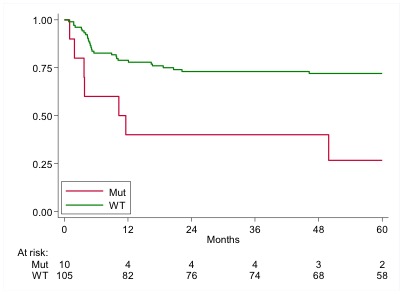
Of 399 DLBCL patients enrolled in FIL-DLCL04, 115 with tumor block available for subsequent analyses were analyzed for TP53 mutation; no selection bias was observed between the 115 cases and the whole FIL-DLCL04 study population. Fifty-five of 115 patients (48%) received R-HDC+ASCT upfront, as for randomization arm; 22 (19%) had bone marrow involvement; 41 (53%) were GCB, 23 (29%) ABC and 14 (18%) unclassified; 20 (19%) were double-expressor (DEL) for MYC and BCL2 in IHC and 8 (8%) double-hit (DHL) for Myc and Bcl2 in FISH. Regarding TP53 status, 105 (91%) were wild type and 10 (9%) mutated; of 10 cases with TP53 mutated, 5 were DEL, 2 DHL and 2 ABC. At a median follow-up of 72 months, 5-years FFS for TP53 mutated versus wild type were 27% (95% CI: 48-56) and 72% (95% CI: 62-80), respectively with a crude hazard ratio (HR) of 3.57 (95% CI: 1.56-8.17), p 0.003, an aHR (clinical) of 2.02 (95% CI: 0.75-5.44), p 0.165 and an aHR (biological) of 2.06 (0.70-6.11), p 0.190. (Figure 1). Five-years OS for TP53 mutated versus Wild type were 37% (95% CI: 10-66) and 84% (95% CI: 75-89), respectively; HR: 4.88 (95% CI: 1.91-12.42), p 0.001, aHR (clinical): 3.26 (95% CI: 0.98-10.9), p 0.055 and aHR (biological) 4.10 (0.89-18.86), p 0.070.
Conclusion
In this series of young patients with high risk DLBCL, TP53 disruption by gene mutation identifies a very poor prognosis subgroup with a dismal FFS and OS, irrespective to IHC, FISH and COO profile. Likewise other hematological malignancies, TP53 disruption is a negative prognostic factor also in DLBCL and these patients require different treatment with innovative drugs.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Diffuse large B cell lymphoma, High dose therapy, MYC, P53
Abstract: S1549
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 09:00 - 09:15
Location: Room A1
Background
The Italian phase III randomized study FIL-DLCL04 (Chiappella et al, Lancet Oncol 2017) showed that an abbreviated Rituximab-CHOP dose-dense chemotherapy followed by consolidation with Rituximab-high dose chemotherapy and autologous stem cell transplantation (R-HDC+ASCT) compared to a full course of R-CHOP dose-dense chemotherapy without consolidation, determined an advantage in term of failure-free survival (FFS) but no difference in overall survival (OS) in young patients with untreated diffuse-large B-cell lymphomas (DLBCL) at high-risk. The prognostic role of TP53 is well-known in chronic lymphocytic leukaemia, but it has not yet been established in DLBCL.
Aims
Aim of this analysis was to correlate TP53 mutations, cell of origin (COO) profile and the presence of biomarkers (MYC, BCL2), with OS and FFS.
Methods
From 2005 to 2010, 399 young untreated DLBCL at poor-risk, were enrolled in FIL-DLCL04 and randomized to receive R-HDC+ASCT in 199 and R-dose-dense in 200 (NCT00499018). TP53 disruption by gene mutation was analyzed by Sanger DNA sequencing. COO classification as germinal center (GCB), activated B-cell (ABC) and unclassified was based on gene-expression profiling using the NanoString research use only lymphoma subtyping test. BCL2, MYC and TP53 were studied in immunohistochemistry (IHC); cases were deemed positive if at least 50%, 40% and 30% of lymphoma cells were stained with BCL2, c-MYC and TP53 antibodies, respectively. Bcl2 and Myc translocations, copy gains and aberrations were tested by fluorescent in situ hybridization (FISH). OS and FFS were analyzed; a crude hazard ratio (HR) and an adjusted HR (aHR) for clinical characteristics (age, treatment, gender, age-adjusted International Prognostic Index, performance status, bone marrow involvement) and for biological factors (double-expressors, double-hit, COO profile) were calculated.
Results
Of 399 DLBCL patients enrolled in FIL-DLCL04, 115 with tumor block available for subsequent analyses were analyzed for TP53 mutation; no selection bias was observed between the 115 cases and the whole FIL-DLCL04 study population. Fifty-five of 115 patients (48%) received R-HDC+ASCT upfront, as for randomization arm; 22 (19%) had bone marrow involvement; 41 (53%) were GCB, 23 (29%) ABC and 14 (18%) unclassified; 20 (19%) were double-expressor (DEL) for MYC and BCL2 in IHC and 8 (8%) double-hit (DHL) for Myc and Bcl2 in FISH. Regarding TP53 status, 105 (91%) were wild type and 10 (9%) mutated; of 10 cases with TP53 mutated, 5 were DEL, 2 DHL and 2 ABC. At a median follow-up of 72 months, 5-years FFS for TP53 mutated versus wild type were 27% (95% CI: 48-56) and 72% (95% CI: 62-80), respectively with a crude hazard ratio (HR) of 3.57 (95% CI: 1.56-8.17), p 0.003, an aHR (clinical) of 2.02 (95% CI: 0.75-5.44), p 0.165 and an aHR (biological) of 2.06 (0.70-6.11), p 0.190. (Figure 1). Five-years OS for TP53 mutated versus Wild type were 37% (95% CI: 10-66) and 84% (95% CI: 75-89), respectively; HR: 4.88 (95% CI: 1.91-12.42), p 0.001, aHR (clinical): 3.26 (95% CI: 0.98-10.9), p 0.055 and aHR (biological) 4.10 (0.89-18.86), p 0.070.

Conclusion
In this series of young patients with high risk DLBCL, TP53 disruption by gene mutation identifies a very poor prognosis subgroup with a dismal FFS and OS, irrespective to IHC, FISH and COO profile. Likewise other hematological malignancies, TP53 disruption is a negative prognostic factor also in DLBCL and these patients require different treatment with innovative drugs.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Diffuse large B cell lymphoma, High dose therapy, MYC, P53


