
Contributions
Abstract: S812
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 12:15 - 12:30
Location: Room A2
Background
Ruxolitinib (RUX) is effective in controlling symptoms and organomegaly in patients with myelofibrosis (MF). Combination with azacitidine (AZA) may further improve its efficacy.
Aims
To evalute efficacy and safety of RUX and AZA combination.
Methods
This was a single insitutional, phase 2 study. RUX 15 or 20 mg orally twice daily was given continuously since cycle 1. AZA 25 to 75 mg/m2 on days 1-5 of each 28-day cycle was added starting cycle 4. Responses were assessed per International Working Group for Myelofibrosis Research and Treatment 2013 criteria (IWG-MRT).
Results
Forty nine patients were enrolled on study between 03/2013 and 08/2017.
After median follow-up of 18.5+ months (range, 1-56+); 14 patients (29%) remain on therapy with a median overall follow-up of 28+ months. 41 patients (84%) received AZA on study, with a median of 15 cycles (range, 1-52).
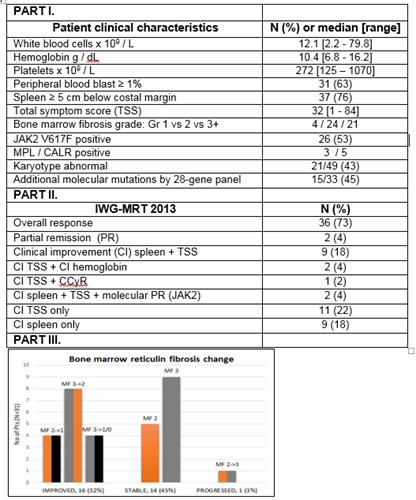
Median age was 66 years (range, 48-87), 37 patients (76%) had int-2/high DIPSS score, 37 (76%) had spleen ≥5cm, and 26 (53%) were JAK2V617F positive (Figure, Part I). Twenty six patients (53%) were previously treated.
Thirty-six patients (73%) achieved an IWG-MRT 2013 objective response (Figure, Part II). Median time to response was 1.7 months (range, 0.7-19). Eight responses (22% of responders) occurred after the addition of AZA with median time to response from start of AZA of 4 months (range, 1-16.5).
In total, 25 (68%) and 22 (59%) patients had palpable spleen reduction by > 50% at any time on study, and at week 24, respectively. Spleen responses occurred after AZA introduction in 6 patients with a median time of 1.8 months from start of AZA. Mean percentage spleen reduction by palpation was 73% (95% CI 59-86%) and 63% (95% CI 25-100%) at any time on study and at week 24, respectively. JAK2V617F allele reduction was noted in 14 (82%) of 17 evaluable patients, including > 50% reduction in 3 patients (18%).
Thirty-one patients (63%) had bone marrow biopsies available for sequential evaluation. Sixteen patients (52%) had a documented improvement in bone marrow fibrosis by at least one grade per European grading (EUMNET) including 4 (13%) by two grades or more, with median time to first documented fibrosis improvement of 12 months (range, 6-18). Additionally, 12 (48%) and 9 (41%) achieved improvement in initially abnormal collagen (n=25) or osteosclerosis (n=22), respectively (Figure, Part III).
Overall, 31 patients (63%) experienced grade 3/4 toxicity while on therapy. New onset of G3/4 anemia, thrombocytopenia and neutropenia occurred in 33%, 24% and 22% of patients, respectively. Only 4 (8%) discontinued therapy due to toxicities.
The most common reasons for therapy discontinuation were elective stem cell transplantation (n=12), and uncontrolled disease (n=8), including progression to AML in 4 patients.
Conclusion
Concomitant RUX with AZA was well tolerated with overall IWG-MRT response rate of 73%, including >50% spleen length reduction in 59% of patients at week 24. Moreover, 52% of patients achieved an independent pathologist committee reviewed, objective improvements in bone marrow fibrosis grade with a median time to improvement of 12 months, which compares favorably to single RUX. ClinicalTrials.gov Identifier: NCT01787487
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Azacitidine, Myelofibrosis, Ruxolitinib
Abstract: S812
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 12:15 - 12:30
Location: Room A2
Background
Ruxolitinib (RUX) is effective in controlling symptoms and organomegaly in patients with myelofibrosis (MF). Combination with azacitidine (AZA) may further improve its efficacy.
Aims
To evalute efficacy and safety of RUX and AZA combination.
Methods
This was a single insitutional, phase 2 study. RUX 15 or 20 mg orally twice daily was given continuously since cycle 1. AZA 25 to 75 mg/m2 on days 1-5 of each 28-day cycle was added starting cycle 4. Responses were assessed per International Working Group for Myelofibrosis Research and Treatment 2013 criteria (IWG-MRT).
Results
Forty nine patients were enrolled on study between 03/2013 and 08/2017.
After median follow-up of 18.5+ months (range, 1-56+); 14 patients (29%) remain on therapy with a median overall follow-up of 28+ months. 41 patients (84%) received AZA on study, with a median of 15 cycles (range, 1-52).
Median age was 66 years (range, 48-87), 37 patients (76%) had int-2/high DIPSS score, 37 (76%) had spleen ≥5cm, and 26 (53%) were JAK2V617F positive (Figure, Part I). Twenty six patients (53%) were previously treated.
Thirty-six patients (73%) achieved an IWG-MRT 2013 objective response (Figure, Part II). Median time to response was 1.7 months (range, 0.7-19). Eight responses (22% of responders) occurred after the addition of AZA with median time to response from start of AZA of 4 months (range, 1-16.5).
In total, 25 (68%) and 22 (59%) patients had palpable spleen reduction by > 50% at any time on study, and at week 24, respectively. Spleen responses occurred after AZA introduction in 6 patients with a median time of 1.8 months from start of AZA. Mean percentage spleen reduction by palpation was 73% (95% CI 59-86%) and 63% (95% CI 25-100%) at any time on study and at week 24, respectively. JAK2V617F allele reduction was noted in 14 (82%) of 17 evaluable patients, including > 50% reduction in 3 patients (18%).
Thirty-one patients (63%) had bone marrow biopsies available for sequential evaluation. Sixteen patients (52%) had a documented improvement in bone marrow fibrosis by at least one grade per European grading (EUMNET) including 4 (13%) by two grades or more, with median time to first documented fibrosis improvement of 12 months (range, 6-18). Additionally, 12 (48%) and 9 (41%) achieved improvement in initially abnormal collagen (n=25) or osteosclerosis (n=22), respectively (Figure, Part III).
Overall, 31 patients (63%) experienced grade 3/4 toxicity while on therapy. New onset of G3/4 anemia, thrombocytopenia and neutropenia occurred in 33%, 24% and 22% of patients, respectively. Only 4 (8%) discontinued therapy due to toxicities.
The most common reasons for therapy discontinuation were elective stem cell transplantation (n=12), and uncontrolled disease (n=8), including progression to AML in 4 patients.

Conclusion
Concomitant RUX with AZA was well tolerated with overall IWG-MRT response rate of 73%, including >50% spleen length reduction in 59% of patients at week 24. Moreover, 52% of patients achieved an independent pathologist committee reviewed, objective improvements in bone marrow fibrosis grade with a median time to improvement of 12 months, which compares favorably to single RUX. ClinicalTrials.gov Identifier: NCT01787487
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Azacitidine, Myelofibrosis, Ruxolitinib


