
Contributions
Abstract: S130
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 11:45 - 12:00
Location: Room A7
Background
Inhibition of Janus-kinase 1/2 (JAK1/2) is a mainstay to treat myeloproliferative neoplasms (MPN). Besides driving MPN, the JAK-STAT pathway is involved in the development of malignant lymphoma. Recent reports point towards a slightly increased risk of lymphoid neoplasms in MPN patients with JAK2 V617F mutations. Moreover, sporadic cases of diffuse large B-cell lymphomas (DLBCL) have been reported in patients with MPN under ruxolitinib treatment. The frequency and potential causes of lymphomas under JAK2 inhibition remain unclear.
Aims
We aimed at identifying the global frequency and the potential causes of aggressive B-cell lymphomas under JAK2 inhibition as well as at early diagnosing patients at risk.
Methods
626 MPN patients (557 with conventional, 59 with JAK1/2 inhibitor treatment) from Vienna and 929 (872 vs 57) from Paris were included in the study. Immunoglobulin rearrangement (IgR) and MPN associated mutations were tested by PCR. IGHV–D–J sequence was assessed by Sanger sequencing according to ERIC recommendations.11 For the detection of BCL2/IGH gene rearrangements, an Identiclone BCL2/JH Translocation Assay Gel Detection Kit was applied according to the manufacturer's instructions. Targeted next generation sequencing was provided by Foundation OneTM Heme (Roche Austria GmbH, Vienna).
Results
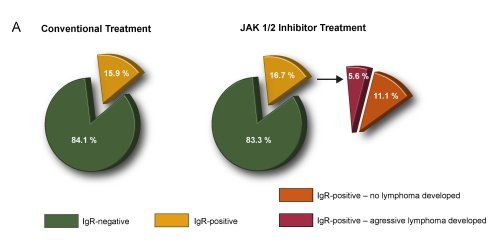
16.7% of JAK 1/2 inhibitor (N=54) and 15.9% of 44 age- and sex-matched conventionally treated patients were positively tested for IgR in the bone marrow of patients with myelofibrosis (MF) (Fig. 1).
In the Viennese cohort, 4 out of 69 (5.8%) developed aggressive lymphomas upon JAK1/2 inhibitor treatment while only 2 lymphomas evolved in 557 patients (0.36%) without inhibitor (Odds ratio (OR) 16, 95% confidence interval (95%CI) 3 to 87; p=0.0017). These results have been validated in an independent cohort from Paris (5.51 vs 0.23%, OR 15, 95%CI 2 - 92, p=0.0205). The median time from start of JAK1/2 inhibitor-treatment to lymphoma diagnosis was 25 months (range 13-35 months). Subgroup analysis of 216 patients with primary myelofibrosis (31 with and 185 without JAK1/2 inhibitor therapy) revealed 3 DLBCL (9.68%) under ruxolitinib and only one (0.54%) in the controls (OR 19, 95%CI 2 - 196, p=0.01). Results remained unchanged after adjustment for age (OR 21, 95%CI 2-218) or sex (OR 25, 95%CI 2-266). 3 of 4 JAK 1/2 inhibitor associated lymphomas were IgR-positive as long as 6 years before overt lymphoma and preceded JAK1/2 inhibition. From one patient no material was available. Sequencing verified clonal identity in 2 patients. The effects of JAK1/2 inhibition were mirrored in Stat1-/- mice: 16/24 mice developed a spontaneous myeloid hyperplasia with the concomitant presence of aberrant B-cells. Transplantations of bone marrow from diseased mice unmasked the outgrowth of a malignant B-cell clone evolving into aggressive B-cell leukemia-lymphoma.
Conclusion
Our results indicate that clonal B-cells are detectable in 15-17% of MF patients. Aggressive lymphomas during JAK1/2 inhibitor treatment occur with increased frequency, have uniform clinic-pathological features and arise from a pre-existent B-cell clone. Early detection of IgR, at the time point of MPN diagnosis, offers the opportunity to determine patients at risk.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Janus Kinase inhibitor, lymphoma, Myelofibrosis, STAT1
Abstract: S130
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 11:45 - 12:00
Location: Room A7
Background
Inhibition of Janus-kinase 1/2 (JAK1/2) is a mainstay to treat myeloproliferative neoplasms (MPN). Besides driving MPN, the JAK-STAT pathway is involved in the development of malignant lymphoma. Recent reports point towards a slightly increased risk of lymphoid neoplasms in MPN patients with JAK2 V617F mutations. Moreover, sporadic cases of diffuse large B-cell lymphomas (DLBCL) have been reported in patients with MPN under ruxolitinib treatment. The frequency and potential causes of lymphomas under JAK2 inhibition remain unclear.
Aims
We aimed at identifying the global frequency and the potential causes of aggressive B-cell lymphomas under JAK2 inhibition as well as at early diagnosing patients at risk.
Methods
626 MPN patients (557 with conventional, 59 with JAK1/2 inhibitor treatment) from Vienna and 929 (872 vs 57) from Paris were included in the study. Immunoglobulin rearrangement (IgR) and MPN associated mutations were tested by PCR. IGHV–D–J sequence was assessed by Sanger sequencing according to ERIC recommendations.11 For the detection of BCL2/IGH gene rearrangements, an Identiclone BCL2/JH Translocation Assay Gel Detection Kit was applied according to the manufacturer's instructions. Targeted next generation sequencing was provided by Foundation OneTM Heme (Roche Austria GmbH, Vienna).
Results
16.7% of JAK 1/2 inhibitor (N=54) and 15.9% of 44 age- and sex-matched conventionally treated patients were positively tested for IgR in the bone marrow of patients with myelofibrosis (MF) (Fig. 1).
In the Viennese cohort, 4 out of 69 (5.8%) developed aggressive lymphomas upon JAK1/2 inhibitor treatment while only 2 lymphomas evolved in 557 patients (0.36%) without inhibitor (Odds ratio (OR) 16, 95% confidence interval (95%CI) 3 to 87; p=0.0017). These results have been validated in an independent cohort from Paris (5.51 vs 0.23%, OR 15, 95%CI 2 - 92, p=0.0205). The median time from start of JAK1/2 inhibitor-treatment to lymphoma diagnosis was 25 months (range 13-35 months). Subgroup analysis of 216 patients with primary myelofibrosis (31 with and 185 without JAK1/2 inhibitor therapy) revealed 3 DLBCL (9.68%) under ruxolitinib and only one (0.54%) in the controls (OR 19, 95%CI 2 - 196, p=0.01). Results remained unchanged after adjustment for age (OR 21, 95%CI 2-218) or sex (OR 25, 95%CI 2-266). 3 of 4 JAK 1/2 inhibitor associated lymphomas were IgR-positive as long as 6 years before overt lymphoma and preceded JAK1/2 inhibition. From one patient no material was available. Sequencing verified clonal identity in 2 patients. The effects of JAK1/2 inhibition were mirrored in Stat1-/- mice: 16/24 mice developed a spontaneous myeloid hyperplasia with the concomitant presence of aberrant B-cells. Transplantations of bone marrow from diseased mice unmasked the outgrowth of a malignant B-cell clone evolving into aggressive B-cell leukemia-lymphoma.

Conclusion
Our results indicate that clonal B-cells are detectable in 15-17% of MF patients. Aggressive lymphomas during JAK1/2 inhibitor treatment occur with increased frequency, have uniform clinic-pathological features and arise from a pre-existent B-cell clone. Early detection of IgR, at the time point of MPN diagnosis, offers the opportunity to determine patients at risk.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Janus Kinase inhibitor, lymphoma, Myelofibrosis, STAT1


