
Contributions
Abstract: S114
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:30 - 12:45
Location: Room A2
Background
Front-line multi-agent chemotherapy cures the majority of patients (pts) with classical Hodgkin lymphoma (cHL). However, pts with advanced-stage (AS) cHL have suboptimal outcomes and the high toxicity of intensive regimens limits their use in elderly/frail pts. Programmed death-1 (PD-1) ligands are commonly overexpressed in cHL and may contribute to an immunosuppressive tumor environment (Roemer MG et al. J Clin Oncol 2016;34:2690–7). Nivolumab, an immune checkpoint inhibitor targeting PD-1, enhances T-cell activation and has shown frequent and durable responses in pts with relapsed/refractory cHL after failure of autologous hematopoietic cell transplantation (Armand P et al. J Clin Oncol 2018;in press). Addition of nivolumab to chemotherapy may therefore be a promising treatment (Tx) strategy for newly diagnosed (ND) AS cHL.
Aims
To assess the safety and efficacy of nivolumab plus doxorubicin, vinblastine and dacarbazine (N-AVD) in pts with ND AS cHL.
Methods
In CheckMate 205 Cohort D (NCT02181738), pts ≥18 y of age with ND AS (stage IIB with unfavorable risk factors, III, or IV) cHL received 4 doses of nivolumab monotherapy (monoTx; 240 mg IV every 2 wk) followed by N-AVD combotherapy (comboTx; nivolumab 240 mg IV) for 6 cycles (12 doses), after informed consent. Primary endpoint was the proportion of pts with grade (G) 3+ Tx-related adverse events (TRAEs) ≤30 d after last dose. Secondary endpoints included complete remission (CR) rate (International Working Group 2007 criteria); exploratory endpoints included discontinuation rate, objective response rate (ORR, per Independent Radiology Review Committee [IRC]) at end of monoTx, after 2 combocycles, and at end of Tx (EOT), and modified progression-free survival (mPFS; time to progression, death, or first subsequent systemic therapy in pts without CR at EOT).
Results
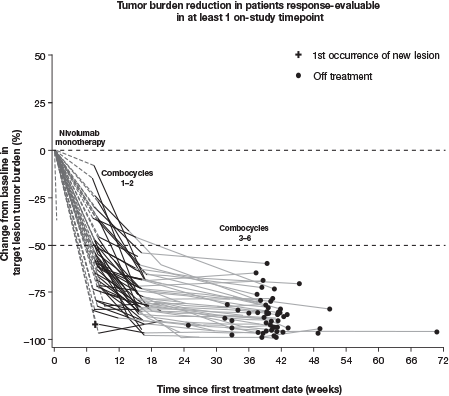
At database lock (Oct 12, 2017), 51 pts had been treated; median follow-up (FU) was 11 mo. At baseline (BL), median age was 37 y and 31% of pts had bulky disease; at diagnosis, 57% had stage IV disease and 80% had B symptoms. MonoTx was completed by 49/51 (96%) pts and comboTx by 45/50 (90%); 1/5 pts who did not complete comboTx was lost to follow-up. 30 pts (59%) experienced a G3–4 TRAE, including neutropenia/decreased neutrophil count in 25 (49%) and febrile neutropenia (FN) in 5 (10%). 30 pts (59%) received growth factors (GFs), all during comboTx; 90% of GF use was secondary prophylaxis. 8 pts (16%) experienced Tx-related infections (excluding FN). No pneumonitis was reported and median change from BL in hemoglobin-corrected diffusing capacity for carbon monoxide was −3% predicted. The most common G3–4 immune-mediated AE was hepatitis (2 pts; 4%). 4 pts (8%) discontinued due to an AE, 1 G3–4. No G5 TRAEs occurred ≤30 d from last dose; 1 pt (age 68 y) died 38 d after last dose due to study drug toxicity. At EOT, ORR in the ITT population was 84% (67% CR) per IRC and 84% (80% CR) per investigator; 7/12 response-evaluable pts without CR per IRC were deemed in CR per investigator. Nearly all response-evaluable pts showed >50% reduction in tumor burden (Figure). Median (range) time to response was 2 (2–5) mo; with a minimum FU of 9 mo, 9-mo mPFS was 94% (95% CI 82, 98).
Conclusion
Nivolumab followed by N-AVD was well tolerated, with a safety profile consistent with previous reports, including a favorable pulmonary toxicity profile. N-AVD had a high ORR, with 67% CR at EOT per IRC. Nivolumab followed by N-AVD warrants further study for ND AS cHL.
Session topic: 17. Hodgkin lymphoma – Clinical
Keyword(s): Cancer immunotherapy, chemotherapy, Clinical Trial, Hodgkin's Lymphoma
Abstract: S114
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:30 - 12:45
Location: Room A2
Background
Front-line multi-agent chemotherapy cures the majority of patients (pts) with classical Hodgkin lymphoma (cHL). However, pts with advanced-stage (AS) cHL have suboptimal outcomes and the high toxicity of intensive regimens limits their use in elderly/frail pts. Programmed death-1 (PD-1) ligands are commonly overexpressed in cHL and may contribute to an immunosuppressive tumor environment (Roemer MG et al. J Clin Oncol 2016;34:2690–7). Nivolumab, an immune checkpoint inhibitor targeting PD-1, enhances T-cell activation and has shown frequent and durable responses in pts with relapsed/refractory cHL after failure of autologous hematopoietic cell transplantation (Armand P et al. J Clin Oncol 2018;in press). Addition of nivolumab to chemotherapy may therefore be a promising treatment (Tx) strategy for newly diagnosed (ND) AS cHL.
Aims
To assess the safety and efficacy of nivolumab plus doxorubicin, vinblastine and dacarbazine (N-AVD) in pts with ND AS cHL.
Methods
In CheckMate 205 Cohort D (NCT02181738), pts ≥18 y of age with ND AS (stage IIB with unfavorable risk factors, III, or IV) cHL received 4 doses of nivolumab monotherapy (monoTx; 240 mg IV every 2 wk) followed by N-AVD combotherapy (comboTx; nivolumab 240 mg IV) for 6 cycles (12 doses), after informed consent. Primary endpoint was the proportion of pts with grade (G) 3+ Tx-related adverse events (TRAEs) ≤30 d after last dose. Secondary endpoints included complete remission (CR) rate (International Working Group 2007 criteria); exploratory endpoints included discontinuation rate, objective response rate (ORR, per Independent Radiology Review Committee [IRC]) at end of monoTx, after 2 combocycles, and at end of Tx (EOT), and modified progression-free survival (mPFS; time to progression, death, or first subsequent systemic therapy in pts without CR at EOT).
Results
At database lock (Oct 12, 2017), 51 pts had been treated; median follow-up (FU) was 11 mo. At baseline (BL), median age was 37 y and 31% of pts had bulky disease; at diagnosis, 57% had stage IV disease and 80% had B symptoms. MonoTx was completed by 49/51 (96%) pts and comboTx by 45/50 (90%); 1/5 pts who did not complete comboTx was lost to follow-up. 30 pts (59%) experienced a G3–4 TRAE, including neutropenia/decreased neutrophil count in 25 (49%) and febrile neutropenia (FN) in 5 (10%). 30 pts (59%) received growth factors (GFs), all during comboTx; 90% of GF use was secondary prophylaxis. 8 pts (16%) experienced Tx-related infections (excluding FN). No pneumonitis was reported and median change from BL in hemoglobin-corrected diffusing capacity for carbon monoxide was −3% predicted. The most common G3–4 immune-mediated AE was hepatitis (2 pts; 4%). 4 pts (8%) discontinued due to an AE, 1 G3–4. No G5 TRAEs occurred ≤30 d from last dose; 1 pt (age 68 y) died 38 d after last dose due to study drug toxicity. At EOT, ORR in the ITT population was 84% (67% CR) per IRC and 84% (80% CR) per investigator; 7/12 response-evaluable pts without CR per IRC were deemed in CR per investigator. Nearly all response-evaluable pts showed >50% reduction in tumor burden (Figure). Median (range) time to response was 2 (2–5) mo; with a minimum FU of 9 mo, 9-mo mPFS was 94% (95% CI 82, 98).

Conclusion
Nivolumab followed by N-AVD was well tolerated, with a safety profile consistent with previous reports, including a favorable pulmonary toxicity profile. N-AVD had a high ORR, with 67% CR at EOT per IRC. Nivolumab followed by N-AVD warrants further study for ND AS cHL.
Session topic: 17. Hodgkin lymphoma – Clinical
Keyword(s): Cancer immunotherapy, chemotherapy, Clinical Trial, Hodgkin's Lymphoma


