
Contributions
Abstract: S107
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:00 - 12:15
Location: Victoria Hall
Background
Daratumumab (D) plus VMP (D-VMP) prolonged progression-free survival compared with VMP and was well-tolerated in the phase 3 ALCYONE study (NCT02195479).
Aims
We examined the efficacy and safety profiles of D-VMP vs VMP in elderly (≥75 years of age) and non-elderly (<75 years of age) newly diagnosed multiple myeloma patients in ALCYONE.
Methods
Patients were 65 years of age or older or otherwise ineligible for high-dose chemotherapy with autologous stem cell transplantation. Patients received up to nine 6-week VMP cycles (V: 1.3 mg/m2 subcutaneously Days 1, 4, 8, 11, 22, 25, 29, 32 [Cycle 1] and Days 1, 8, 22, 29 [Cycles 2-9]; M: 9 mg/m2 orally and P: 60 mg/m2 orally Days 1-4 [Cycles 1-9]) with or without D (16 mg/kg intravenously QW for Cycle 1, Q3W for Cycles 2-9, and Q4W for Cycles 10+ [post VMP-treatment phase] until progression). Minimal residual disease was assessed by clonoSEQ® assay (Adaptive Biotechnologies).
Results
706 (350 D-VMP; 356 VMP) patients were randomized, including 211 patients ≥75 years of age (104 D-VMP; 107 VMP) and 495 patients <75 years of age (246 D-VMP; 249 VMP). For D-VMP vs VMP, the median duration of study treatment was 14.5 months vs 12.0 months for patients ≥75 years of age and 15.0 months vs 12.0 months for patients <75 years of age, respectively. The cumulative dose of bortezomib was 43.1 mg/m2 and 34.1 mg/m2 with D-VMP and VMP, respectively, for patients ≥75 years of age, and 48.6 mg/m2 and 46.2 mg/m2 with D-VMP and VMP, respectively, for patients <75 years of age.
After median follow-up of 16.5 months, progression-free survival was prolonged with D-VMP vs VMP both in patients ≥75 years of age (median not reached [NR] vs 20.4 months; hazard ratio [HR] 0.53; 95% confidence interval [CI] 0.32-0.85) and patients <75 years of age (median NR vs 17.9 months; HR 0.49; 95% CI 0.36-0.68). Overall response rate (ORR) and ≥complete response (CR) rates were consistently higher for D-VMP vs VMP in patients ≥75 years of age (ORR: 88% vs 70%; ≥CR: 41% vs 24%) and <75 years of age (ORR: 92% vs 76%; ≥CR: 43% vs 25%). Minimal residual disease-negative rates (10–5 threshold) also increased with D-VMP vs VMP in patients ≥75 years of age (24% vs 8%) and <75 years of age (22% vs 6%).
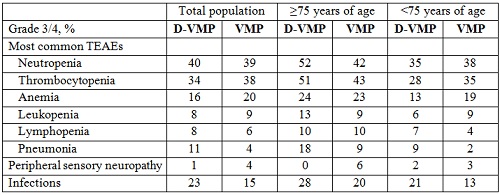
Rates of most common grade 3/4 (≥10%) treatment-emergent adverse events, peripheral sensory neuropathy, and infections are presented in the Table. D-associated infusion-related reactions were 36% (9% grade 3/4) in patients ≥75 years of age and 24% (3% grade 3/4) in patients <75 years of age.
Conclusion
The efficacy of D-VMP vs VMP in patients ≥75 years of age was consistent with the overall study population. Furthermore, D in combination with VMP demonstrated acceptable tolerability regardless of age.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Elderly, Phase III
Abstract: S107
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:00 - 12:15
Location: Victoria Hall
Background
Daratumumab (D) plus VMP (D-VMP) prolonged progression-free survival compared with VMP and was well-tolerated in the phase 3 ALCYONE study (NCT02195479).
Aims
We examined the efficacy and safety profiles of D-VMP vs VMP in elderly (≥75 years of age) and non-elderly (<75 years of age) newly diagnosed multiple myeloma patients in ALCYONE.
Methods
Patients were 65 years of age or older or otherwise ineligible for high-dose chemotherapy with autologous stem cell transplantation. Patients received up to nine 6-week VMP cycles (V: 1.3 mg/m2 subcutaneously Days 1, 4, 8, 11, 22, 25, 29, 32 [Cycle 1] and Days 1, 8, 22, 29 [Cycles 2-9]; M: 9 mg/m2 orally and P: 60 mg/m2 orally Days 1-4 [Cycles 1-9]) with or without D (16 mg/kg intravenously QW for Cycle 1, Q3W for Cycles 2-9, and Q4W for Cycles 10+ [post VMP-treatment phase] until progression). Minimal residual disease was assessed by clonoSEQ® assay (Adaptive Biotechnologies).
Results
706 (350 D-VMP; 356 VMP) patients were randomized, including 211 patients ≥75 years of age (104 D-VMP; 107 VMP) and 495 patients <75 years of age (246 D-VMP; 249 VMP). For D-VMP vs VMP, the median duration of study treatment was 14.5 months vs 12.0 months for patients ≥75 years of age and 15.0 months vs 12.0 months for patients <75 years of age, respectively. The cumulative dose of bortezomib was 43.1 mg/m2 and 34.1 mg/m2 with D-VMP and VMP, respectively, for patients ≥75 years of age, and 48.6 mg/m2 and 46.2 mg/m2 with D-VMP and VMP, respectively, for patients <75 years of age.
After median follow-up of 16.5 months, progression-free survival was prolonged with D-VMP vs VMP both in patients ≥75 years of age (median not reached [NR] vs 20.4 months; hazard ratio [HR] 0.53; 95% confidence interval [CI] 0.32-0.85) and patients <75 years of age (median NR vs 17.9 months; HR 0.49; 95% CI 0.36-0.68). Overall response rate (ORR) and ≥complete response (CR) rates were consistently higher for D-VMP vs VMP in patients ≥75 years of age (ORR: 88% vs 70%; ≥CR: 41% vs 24%) and <75 years of age (ORR: 92% vs 76%; ≥CR: 43% vs 25%). Minimal residual disease-negative rates (10–5 threshold) also increased with D-VMP vs VMP in patients ≥75 years of age (24% vs 8%) and <75 years of age (22% vs 6%).
Rates of most common grade 3/4 (≥10%) treatment-emergent adverse events, peripheral sensory neuropathy, and infections are presented in the Table. D-associated infusion-related reactions were 36% (9% grade 3/4) in patients ≥75 years of age and 24% (3% grade 3/4) in patients <75 years of age.

Conclusion
The efficacy of D-VMP vs VMP in patients ≥75 years of age was consistent with the overall study population. Furthermore, D in combination with VMP demonstrated acceptable tolerability regardless of age.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Elderly, Phase III


