
Contributions
Abstract: S810
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 11:45 - 12:00
Location: Room A2
Background
It is well documented that residual BCR-ABL positive leukemia stem cells are responsible for disease persistence in chronic phase CML (CP-CML), despite TKI therapy. Autophagy is rapidly induced following TKI treatment in vitro in CP-CML. Pharmacological autophagy inhibition, using lysosomotropic chloroquine (CQ), enhances the effect of TKIs in CP-CML, compared to Imatinib (IM) or CQ alone. Here, we report the results of the largest clinical trial of autophagy inhibition [CHOICES (CHlorOquine and Imatinib Combination to Eliminate Stem cells] trial. CP-CML patients in major cytogenetic response (MCyR) with residual disease detectable after at least one year of IM treatment were treated with IM and hydroxychloroquine (HCQ) or IM alone.
Aims
The primary study end-point was the proportion of treatment ‘successes’, defined as patients who had >0.5 log reduction in their 12-month (mth) qPCR level from baseline. The secondary study end-points were the proportion of treatment ‘successes’ at 24 mth, molecular response and progression at 12 and 24 mth, comparison of IM levels between study arms, and the proportion of patients who achieved whole blood HCQ levels >2000ng/ml.
Methods
CHOICES was an international multicentre, two-arm, parallel, randomised phase II trial with a safety run-in, designed to study the safety and efficacy of IM+HCQ. Patients were randomly assigned, using one-to-one allocation, to receive either IM alone, or IM+HCQ. IM was continued at the same dose that was taken prior to trial entry. At the end of 12 cycles, IM was continued. HCQ was initially started at 800mg/day.
Results
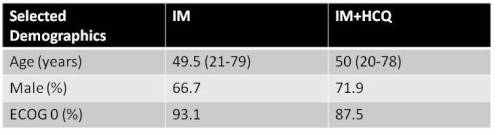
From 2010 to 2014, 62 patients were randomized to IM or IM+HCQ. Demographic data at study entry are listed in table 1. The success rate in the IM+HCQ arm at 12 mnth was 1.2% lower than with IM alone (95% CI 21.1% lower to 18.4% higher; 1-sided p=0.58). MMR was achieved in 80% of IM alone vs 92% in IM+HCQ arm (p =0.21). At 24 mth, the ‘success’ rate in the IM+HCQ arm was 20.8% higher than the IM alone arm (p = 0.059), and MMR was achieved in 79.2% IM and 88% in thr IM+HCQ (p=0.1) group. Within the IM+HCQ experimental arm, HCQ levels were determined over time and analysed by the 12 and 24 mth ‘success’ and ‘failure’ status. There was no correlation between achieving ‘success’ and achieving HCQ concentrations of >2000ng/ml at either time point. During the trial period, 17 adverse events were reported; four were serious adverse reactions: 1 within the IM arm (dyspepsia), and 3 with IM+HCQ. Of these, 1 was unrelated, and 2 had a potential association, namely dyspnea and heart failure. An in vitro assessment of autophagy was performed within the CD34+ stem/progenitor and mononuclear populations from peripheral blood and bone marrow at each time point. Basal autophagy, assessed by LC3B-II levels, was higher in CD34+ stem/progenitor cells compared to mononuclear populations (p=0.002). Additionally, LC3B-II levels at 6 and 12 mth were higher in IM/HCQ arm than the IM arm, suggesting a block in autophagy flow (i.e. inhibition of autophagy-mediated degradation of LC3B-II), although this was not statistically significant.
Conclusion
We confirm that IM+HCQ is a tolerable combination in CP-CML, with significant improvement in overall qPCR levels and MMR at 24 mth. This suggests that there may be a potential long-term benefit of combined therapy on qPCR levels and achievement of deep molecular response. Longer follow-up will be required to confirm this.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, Clinical Trial, Leukemic Stem Cell
Abstract: S810
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 11:45 - 12:00
Location: Room A2
Background
It is well documented that residual BCR-ABL positive leukemia stem cells are responsible for disease persistence in chronic phase CML (CP-CML), despite TKI therapy. Autophagy is rapidly induced following TKI treatment in vitro in CP-CML. Pharmacological autophagy inhibition, using lysosomotropic chloroquine (CQ), enhances the effect of TKIs in CP-CML, compared to Imatinib (IM) or CQ alone. Here, we report the results of the largest clinical trial of autophagy inhibition [CHOICES (CHlorOquine and Imatinib Combination to Eliminate Stem cells] trial. CP-CML patients in major cytogenetic response (MCyR) with residual disease detectable after at least one year of IM treatment were treated with IM and hydroxychloroquine (HCQ) or IM alone.
Aims
The primary study end-point was the proportion of treatment ‘successes’, defined as patients who had >0.5 log reduction in their 12-month (mth) qPCR level from baseline. The secondary study end-points were the proportion of treatment ‘successes’ at 24 mth, molecular response and progression at 12 and 24 mth, comparison of IM levels between study arms, and the proportion of patients who achieved whole blood HCQ levels >2000ng/ml.
Methods
CHOICES was an international multicentre, two-arm, parallel, randomised phase II trial with a safety run-in, designed to study the safety and efficacy of IM+HCQ. Patients were randomly assigned, using one-to-one allocation, to receive either IM alone, or IM+HCQ. IM was continued at the same dose that was taken prior to trial entry. At the end of 12 cycles, IM was continued. HCQ was initially started at 800mg/day.
Results
From 2010 to 2014, 62 patients were randomized to IM or IM+HCQ. Demographic data at study entry are listed in table 1. The success rate in the IM+HCQ arm at 12 mnth was 1.2% lower than with IM alone (95% CI 21.1% lower to 18.4% higher; 1-sided p=0.58). MMR was achieved in 80% of IM alone vs 92% in IM+HCQ arm (p =0.21). At 24 mth, the ‘success’ rate in the IM+HCQ arm was 20.8% higher than the IM alone arm (p = 0.059), and MMR was achieved in 79.2% IM and 88% in thr IM+HCQ (p=0.1) group. Within the IM+HCQ experimental arm, HCQ levels were determined over time and analysed by the 12 and 24 mth ‘success’ and ‘failure’ status. There was no correlation between achieving ‘success’ and achieving HCQ concentrations of >2000ng/ml at either time point. During the trial period, 17 adverse events were reported; four were serious adverse reactions: 1 within the IM arm (dyspepsia), and 3 with IM+HCQ. Of these, 1 was unrelated, and 2 had a potential association, namely dyspnea and heart failure. An in vitro assessment of autophagy was performed within the CD34+ stem/progenitor and mononuclear populations from peripheral blood and bone marrow at each time point. Basal autophagy, assessed by LC3B-II levels, was higher in CD34+ stem/progenitor cells compared to mononuclear populations (p=0.002). Additionally, LC3B-II levels at 6 and 12 mth were higher in IM/HCQ arm than the IM arm, suggesting a block in autophagy flow (i.e. inhibition of autophagy-mediated degradation of LC3B-II), although this was not statistically significant.

Conclusion
We confirm that IM+HCQ is a tolerable combination in CP-CML, with significant improvement in overall qPCR levels and MMR at 24 mth. This suggests that there may be a potential long-term benefit of combined therapy on qPCR levels and achievement of deep molecular response. Longer follow-up will be required to confirm this.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, Clinical Trial, Leukemic Stem Cell


