
Contributions
Abstract: S1548
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 08:45 - 09:00
Location: Room A1
Background
Outcomes for patients (pts) with intermediate-high and high risk diffuse large B-cell lymphoma (DLBCL) after R-CHOP chemotherapy remain suboptimal but no other regimen has improved survival rates. Even less is known about the management of other forms of high grade B-cell lymphoma (HGBL) - double hit lymphoma (DHL) and HGBL not otherwise specified (NOS) - where outcomes are poor with no established standard of care. The phase 2 R-CODOX-M trial was designed to assess the efficacy of adding rituximab to the CODOX-M/IVAC regimen in high risk HGBL. Primary results have previously been presented (McMillan et al, ICML 2015).
Aims
To present outcomes according to pathology subgroups and updated survival for both DLBCL/HGBL and Burkitt lymphoma (BL) cohorts.
Methods
Between 2009 and 2013, 150 pts were registered at 20 UK sites. Eligible pts were aged 18-65 years with stage II-IV untreated DLBCL/HGBL or BL and an International Prognostic Index (IPI) score ≥3. Pts received dose-modified CODOX-M and IVAC (Mead et al, Blood 2008) with the addition of 8 doses of rituximab (375mg/m2). Diagnostic pathology was centrally reviewed by the Leeds Haematological Malignancy Diagnostic Service in 117 (78%) pts and diagnostic reports from central teaching hospitals were reviewed for the remainder. The original site diagnosis was changed from BL to DLBCL/HGBL in 11/36 (30.6%) pts and from DLBCL to BL in 4/114 (3.5%) following central review and FISH analysis. Six pts did not commence treatment and are excluded from further analyses.
Results
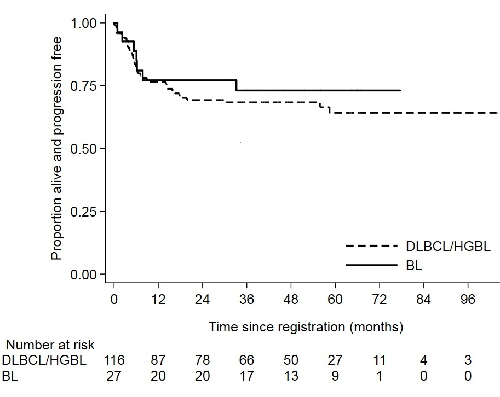
A total of 116 pts are included in the DLBCL/HGBL cohort. Median age was 50 years (range 18–65), IPI score was 3 (n=74; 64%), 4 (n=41; 35%) or 5 (n=1; 1%). Eleven pts (9.5%) had CNS involvement and 62 (53%) had a performance status (PS) ≥2. Cell of origin classification by Hans algorithm was available for 104 pts, of which 57 (55%) were germinal centre B-cell (GCB). FISH data was available for 57 pts, of which 7 (12%) were double/triple hit lymphomas. Five pts had HGBL-NOS (defined as a BL-like phenotype without MYC-R) and 4 pts were indeterminate between HGBL/BL due to failed FISH studies. With a median follow-up of 53 months for the whole DLBCL/HGBL cohort, 3-year progression-free survival (PFS) and overall survival (OS) were 68.4% (95% CI: 59-76.1; see Figure) and 76.2% (95% CI: 76.2 – 67.2), respectively. There was no difference in outcomes according to cell of origin (p=0.73), even excluding those with DHL, HGBL-NOS or indeterminate pathology. Three-year PFS rates were 57.1% for DHL (95% CI: 17.2–83.7) and 63.6% for those with HGBL-NOS/indeterminate histology (95% CI 29.7–84.5). Pts with secondary CNS lymphoma had a 3-year PFS of 72.7% (95% CI: 37.1–90.3). Treatment-related mortality was 4.3%; all were aged >50 years with a PS of 3.
The BL cohort included 27 pts with a median age of 35 years (range 20–64). IPI score was 3 (n=13; 48%) or 4 (n=14; 52%); PS was ≥2 in 16 pts (59%) and 4 (15%) had CNS involvement. Three-year PFS and OS were 73.1% (95% CI: 51.7-86.2; Figure) and 76.4% (95% CI: 54.8–88.7), respectively.
Conclusion
These results demonstrate very good outcomes with R-CODOX-M/R-IVAC treatment in a high risk cohort of DLBCL/HGBL pts, similar to very high risk BL pts. For pts with aggressive HGBL, where urgent treatment is often necessary but accurate rapid diagnostic differentiation can be difficult, R-CODOX-M/R-IVAC is potentially a favourable treatment option offering improved outcomes for all diagnostic groups. Further trials are needed to assess this regimen against standard of care in high risk DLBCL/HGBL.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Burkitt's lymphoma, DLBCL, Rituximab
Abstract: S1548
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 08:45 - 09:00
Location: Room A1
Background
Outcomes for patients (pts) with intermediate-high and high risk diffuse large B-cell lymphoma (DLBCL) after R-CHOP chemotherapy remain suboptimal but no other regimen has improved survival rates. Even less is known about the management of other forms of high grade B-cell lymphoma (HGBL) - double hit lymphoma (DHL) and HGBL not otherwise specified (NOS) - where outcomes are poor with no established standard of care. The phase 2 R-CODOX-M trial was designed to assess the efficacy of adding rituximab to the CODOX-M/IVAC regimen in high risk HGBL. Primary results have previously been presented (McMillan et al, ICML 2015).
Aims
To present outcomes according to pathology subgroups and updated survival for both DLBCL/HGBL and Burkitt lymphoma (BL) cohorts.
Methods
Between 2009 and 2013, 150 pts were registered at 20 UK sites. Eligible pts were aged 18-65 years with stage II-IV untreated DLBCL/HGBL or BL and an International Prognostic Index (IPI) score ≥3. Pts received dose-modified CODOX-M and IVAC (Mead et al, Blood 2008) with the addition of 8 doses of rituximab (375mg/m2). Diagnostic pathology was centrally reviewed by the Leeds Haematological Malignancy Diagnostic Service in 117 (78%) pts and diagnostic reports from central teaching hospitals were reviewed for the remainder. The original site diagnosis was changed from BL to DLBCL/HGBL in 11/36 (30.6%) pts and from DLBCL to BL in 4/114 (3.5%) following central review and FISH analysis. Six pts did not commence treatment and are excluded from further analyses.
Results
A total of 116 pts are included in the DLBCL/HGBL cohort. Median age was 50 years (range 18–65), IPI score was 3 (n=74; 64%), 4 (n=41; 35%) or 5 (n=1; 1%). Eleven pts (9.5%) had CNS involvement and 62 (53%) had a performance status (PS) ≥2. Cell of origin classification by Hans algorithm was available for 104 pts, of which 57 (55%) were germinal centre B-cell (GCB). FISH data was available for 57 pts, of which 7 (12%) were double/triple hit lymphomas. Five pts had HGBL-NOS (defined as a BL-like phenotype without MYC-R) and 4 pts were indeterminate between HGBL/BL due to failed FISH studies. With a median follow-up of 53 months for the whole DLBCL/HGBL cohort, 3-year progression-free survival (PFS) and overall survival (OS) were 68.4% (95% CI: 59-76.1; see Figure) and 76.2% (95% CI: 76.2 – 67.2), respectively. There was no difference in outcomes according to cell of origin (p=0.73), even excluding those with DHL, HGBL-NOS or indeterminate pathology. Three-year PFS rates were 57.1% for DHL (95% CI: 17.2–83.7) and 63.6% for those with HGBL-NOS/indeterminate histology (95% CI 29.7–84.5). Pts with secondary CNS lymphoma had a 3-year PFS of 72.7% (95% CI: 37.1–90.3). Treatment-related mortality was 4.3%; all were aged >50 years with a PS of 3.
The BL cohort included 27 pts with a median age of 35 years (range 20–64). IPI score was 3 (n=13; 48%) or 4 (n=14; 52%); PS was ≥2 in 16 pts (59%) and 4 (15%) had CNS involvement. Three-year PFS and OS were 73.1% (95% CI: 51.7-86.2; Figure) and 76.4% (95% CI: 54.8–88.7), respectively.

Conclusion
These results demonstrate very good outcomes with R-CODOX-M/R-IVAC treatment in a high risk cohort of DLBCL/HGBL pts, similar to very high risk BL pts. For pts with aggressive HGBL, where urgent treatment is often necessary but accurate rapid diagnostic differentiation can be difficult, R-CODOX-M/R-IVAC is potentially a favourable treatment option offering improved outcomes for all diagnostic groups. Further trials are needed to assess this regimen against standard of care in high risk DLBCL/HGBL.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Burkitt's lymphoma, DLBCL, Rituximab


