
Contributions
Abstract: S1562
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 08:30 - 08:45
Location: Victoria Hall
Background
IDH1 and IDH2 mutations occur in approximately 20% of patients with AML, with increasing frequency in older patients. Ivosidenib (AG-120) and enasidenib (AG-221) are oral small-molecule inhibitors of mIDH1 and mIDH2 proteins, respectively, both shown to promote myeloid differentiation. As monotherapies, enasidenib and ivosidenib induced clinical responses in patients (pts) with mIDH relapsed/refractory AML. AZA monotherapy prolonged survival vs conventional care in older pts with newly diagnosed (ND) AML. AZA reduces DNA methylation by inhibiting DNA methyltransferases, and mIDH inhibitors indirectly reduce DNA methylation by suppressing the oncometabolite, 2-HG, and restoring function to α-ketoglutarate-dependent TET family enzymes. In vitro, mIDH inhibitor + AZA combinations enhanced cell differentiation and apoptosis.
Aims
Evaluate clinical outcomes with combination mIDH inhibitors + AZA in older pts with ND-AML in an ongoing phase 1b/2 study (NCT02677922).
Methods
Adult pts with mIDH ND-AML ineligible for intensive treatment (Tx) and ECOG PS scores ≤2 were eligible. Pts received ivosidenib (mIDH1) 500 mg QD or enasidenib (mIDH2) 100 or 200 mg QD, plus SC AZA 75 mg/m2 x 7d, in continuous 28d cycles. Response was defined by modified IWG 2003 AML criteria; overall response rate (ORR) included complete remission (CR), CR with incomplete count recovery (CRi/CRp), partial remission (PR), and morphologic leukemia-free state (MLFS).
Results
At data cutoff (Sep 1, 2017) 17 pts had received ivosidenib 500 mg (n=11) or enasidenib 100 mg (n=3) or 200 mg (n=3) + AZA in the phase 1b portion of the study; 11 pts were ongoing.
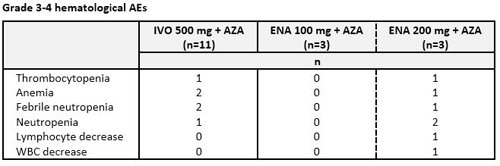
Ivosidenib: Median age was 76 yrs (range 74-82) and 82% of pts had an ECOG PS score of 1. Median number of Tx cycles was 3 (range 1-13). Three pts discontinued Tx, 2 due to progressive disease (PD). AEs in ≥4 pts (any grade) were nausea (n=8), constipation (6), fatigue (5) and diarrhea (4). Grade 3-4 hematological AEs (Table) occurred at similar frequency to what has been reported for AZA alone. Serious AEs in >1 pt were pneumonia and febrile neutropenia (n=2 each). Eight of 11 pts responded: 4 pts attained CR, 1 CRi, 1 PR, and 2 MLFS.
Enasidenib: Median age was 68 yrs (range 65-76) and 83% of pts had an ECOG PS score of 1. Median number of Tx cycles was 9 (1-13). Three pts discontinued Tx, 2 due to PD. Most common AEs (any grade) were nausea and hyperbilirubinemia (n=4 each). Serious AEs in >1 pt were pyrexia, bilirubin increase, pneumonia (n=2 each). Four of 6 pts responded: 2 pts achieved CR, 1 PR, and 1 MLFS.
Conclusion
mIDH inhibitor + AZA regimens were generally well tolerated in these older pts with ND-AML; 65% of pts remained on-study at data cutoff. The most common AEs were grade 1-2 gastrointestinal events and enasidenib-related indirect bilirubin elevations, likely due to off-target inhibition of the UGT1A1 enzyme. Response rates are encouraging. Phase 1b enrollment completed in late 2017; updated data for all 23 ivosidenib and 6 enasidenib pts will be presented, as well as longitudinal changes in mIDH variant allele frequencies. Enrollment continues in the phase 2 portion of this study (enasidenib + AZA) and in the phase 3 AGILE study of ivosidenib+ AZA (NCT03173248).
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Acute Myeloid Leukemia, Azacitidine, Enasidenib, Ivosidenib
Abstract: S1562
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 08:30 - 08:45
Location: Victoria Hall
Background
IDH1 and IDH2 mutations occur in approximately 20% of patients with AML, with increasing frequency in older patients. Ivosidenib (AG-120) and enasidenib (AG-221) are oral small-molecule inhibitors of mIDH1 and mIDH2 proteins, respectively, both shown to promote myeloid differentiation. As monotherapies, enasidenib and ivosidenib induced clinical responses in patients (pts) with mIDH relapsed/refractory AML. AZA monotherapy prolonged survival vs conventional care in older pts with newly diagnosed (ND) AML. AZA reduces DNA methylation by inhibiting DNA methyltransferases, and mIDH inhibitors indirectly reduce DNA methylation by suppressing the oncometabolite, 2-HG, and restoring function to α-ketoglutarate-dependent TET family enzymes. In vitro, mIDH inhibitor + AZA combinations enhanced cell differentiation and apoptosis.
Aims
Evaluate clinical outcomes with combination mIDH inhibitors + AZA in older pts with ND-AML in an ongoing phase 1b/2 study (NCT02677922).
Methods
Adult pts with mIDH ND-AML ineligible for intensive treatment (Tx) and ECOG PS scores ≤2 were eligible. Pts received ivosidenib (mIDH1) 500 mg QD or enasidenib (mIDH2) 100 or 200 mg QD, plus SC AZA 75 mg/m2 x 7d, in continuous 28d cycles. Response was defined by modified IWG 2003 AML criteria; overall response rate (ORR) included complete remission (CR), CR with incomplete count recovery (CRi/CRp), partial remission (PR), and morphologic leukemia-free state (MLFS).
Results
At data cutoff (Sep 1, 2017) 17 pts had received ivosidenib 500 mg (n=11) or enasidenib 100 mg (n=3) or 200 mg (n=3) + AZA in the phase 1b portion of the study; 11 pts were ongoing.
Ivosidenib: Median age was 76 yrs (range 74-82) and 82% of pts had an ECOG PS score of 1. Median number of Tx cycles was 3 (range 1-13). Three pts discontinued Tx, 2 due to progressive disease (PD). AEs in ≥4 pts (any grade) were nausea (n=8), constipation (6), fatigue (5) and diarrhea (4). Grade 3-4 hematological AEs (Table) occurred at similar frequency to what has been reported for AZA alone. Serious AEs in >1 pt were pneumonia and febrile neutropenia (n=2 each). Eight of 11 pts responded: 4 pts attained CR, 1 CRi, 1 PR, and 2 MLFS.
Enasidenib: Median age was 68 yrs (range 65-76) and 83% of pts had an ECOG PS score of 1. Median number of Tx cycles was 9 (1-13). Three pts discontinued Tx, 2 due to PD. Most common AEs (any grade) were nausea and hyperbilirubinemia (n=4 each). Serious AEs in >1 pt were pyrexia, bilirubin increase, pneumonia (n=2 each). Four of 6 pts responded: 2 pts achieved CR, 1 PR, and 1 MLFS.

Conclusion
mIDH inhibitor + AZA regimens were generally well tolerated in these older pts with ND-AML; 65% of pts remained on-study at data cutoff. The most common AEs were grade 1-2 gastrointestinal events and enasidenib-related indirect bilirubin elevations, likely due to off-target inhibition of the UGT1A1 enzyme. Response rates are encouraging. Phase 1b enrollment completed in late 2017; updated data for all 23 ivosidenib and 6 enasidenib pts will be presented, as well as longitudinal changes in mIDH variant allele frequencies. Enrollment continues in the phase 2 portion of this study (enasidenib + AZA) and in the phase 3 AGILE study of ivosidenib+ AZA (NCT03173248).
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Acute Myeloid Leukemia, Azacitidine, Enasidenib, Ivosidenib


