
Contributions
Abstract: S1559
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 09:00 - 09:15
Location: Room A4
Background
Many patients (pts) with lower-risk myelodysplastic syndrome (MDS) experience anemia. Transfusion is a treatment option; however, it is often associated with iron overload and other adverse effects. Erythropoiesis-stimulating agents (ESAs) are recommended to treat anemia in pts with MDS; however, long-acting ESAs, such as darbepoetin alfa (DAR), have limited phase 3 data supporting this indication.
Aims
To assess long-term follow-up (LTFU) and survival in anemic pts with low or int-1–risk MDS who received DAR or placebo (PBO).
Methods
This is the final analysis from the LTFU of a phase 3, randomized (2:1), PBO-controlled trial (NCT01362140) that enrolled European pts with anemia and low/int‑1 IPSS–risk MDS. Pts had not previously received ESAs or disease-modifying agents for MDS. Primary analysis results have been published (Platzbecker et al Leukemia 2017). The study comprised a 3-wk screening period and a 24-wk double-blind (DB) treatment period; pts subsequently crossed over to a 48-wk open-label (OL) treatment phase and then LTFU for up to 3 years on study during which physicians could prescribe any therapy. Pts received DAR 500 µg Q3W, adjusted to maintain hemoglobin at 11–12 g/dL. Endpoints were incidence of RBC transfusion and IWG 2006 erythroid response in the DB and OL phases, and survival and disease progression to acute myeloid leukemia (AML) in LTFU.
Results
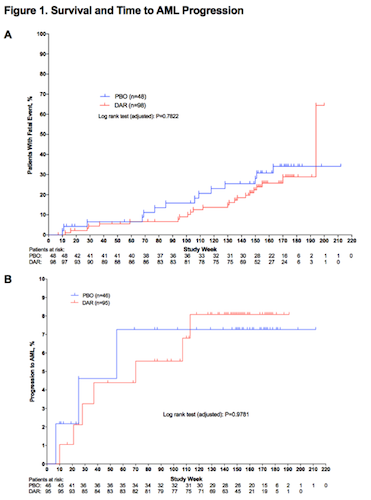
98 pts received DAR and 48 received PBO. 55% were men and mean age was 72 years. In the DB phase, transfusion rates were 36% for DAR and 59% for PBO; erythroid response rates were 15% for DAR vs 0% for PBO (P=0.016). There were 41 deaths during the study; 27 pts (28%) in the DAR group and 14 pts (29%) in the PBO group. Most pts died during LTFU (DAR, 93% and PBO, 79%) and were int-1 at baseline (DAR, 56% and PBO, 71%). Survival curves are presented (Fig 1A). 10 pts progressed to AML (Table). Median time to AML progression was not reached in either treatment group (Fig 1B).
| Treatment group (Pt response per MDSAC) | IPSS at baseline | MDS duration at baseline, days | Highest DAR dose | Day of first progression to AML | Study period |
| PBO | int-1 | 82 | 0 | 46 | DB |
| PBO | int-1 | 208 | 0 | 175 | LTFU |
| PBO | int-1 | 91 | Q3W | 379 | LTFU |
| DAR | int-1 | 61 | Q3W | 64 | DB |
| DAR | low | 247 | Q3W | 146 | DB |
| DAR | int-1 | 85 | Q3W | 195 | LTFU |
| DAR | int-1 | 76 | Q3W | 254 | OL |
| DAR (pt had response from wks 37–45) | int-1 | 80 | Q2W | 489 | OL |
| DAR | int-1 | 191 | Q2W | 788 | LTFU |
| DAR (pt had response from wks 10–37) | low | 296 | Q2W | 743 | LTFU |
Conclusion
In this study, there were no differences in rates of death (mostly reported in LTFU) or AML between DAR and PBO arms. Pts with int-1 risk MDS had worse prognosis. Findings are limited because the study was not powered to detect differences in survival and pts could receive any therapy of physician’s choice in LTFU. In conclusion, DAR is a safe treatment and lowered transfusion rates associated with anemia of low/int-1 risk MDS.
Session topic: 10. Myelodysplastic syndromes – Clinical
Keyword(s): Anemia, Darbepoetin alfa, MDS
Abstract: S1559
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 09:00 - 09:15
Location: Room A4
Background
Many patients (pts) with lower-risk myelodysplastic syndrome (MDS) experience anemia. Transfusion is a treatment option; however, it is often associated with iron overload and other adverse effects. Erythropoiesis-stimulating agents (ESAs) are recommended to treat anemia in pts with MDS; however, long-acting ESAs, such as darbepoetin alfa (DAR), have limited phase 3 data supporting this indication.
Aims
To assess long-term follow-up (LTFU) and survival in anemic pts with low or int-1–risk MDS who received DAR or placebo (PBO).
Methods
This is the final analysis from the LTFU of a phase 3, randomized (2:1), PBO-controlled trial (NCT01362140) that enrolled European pts with anemia and low/int‑1 IPSS–risk MDS. Pts had not previously received ESAs or disease-modifying agents for MDS. Primary analysis results have been published (Platzbecker et al Leukemia 2017). The study comprised a 3-wk screening period and a 24-wk double-blind (DB) treatment period; pts subsequently crossed over to a 48-wk open-label (OL) treatment phase and then LTFU for up to 3 years on study during which physicians could prescribe any therapy. Pts received DAR 500 µg Q3W, adjusted to maintain hemoglobin at 11–12 g/dL. Endpoints were incidence of RBC transfusion and IWG 2006 erythroid response in the DB and OL phases, and survival and disease progression to acute myeloid leukemia (AML) in LTFU.
Results
98 pts received DAR and 48 received PBO. 55% were men and mean age was 72 years. In the DB phase, transfusion rates were 36% for DAR and 59% for PBO; erythroid response rates were 15% for DAR vs 0% for PBO (P=0.016). There were 41 deaths during the study; 27 pts (28%) in the DAR group and 14 pts (29%) in the PBO group. Most pts died during LTFU (DAR, 93% and PBO, 79%) and were int-1 at baseline (DAR, 56% and PBO, 71%). Survival curves are presented (Fig 1A). 10 pts progressed to AML (Table). Median time to AML progression was not reached in either treatment group (Fig 1B).
| Treatment group (Pt response per MDSAC) | IPSS at baseline | MDS duration at baseline, days | Highest DAR dose | Day of first progression to AML | Study period |
| PBO | int-1 | 82 | 0 | 46 | DB |
| PBO | int-1 | 208 | 0 | 175 | LTFU |
| PBO | int-1 | 91 | Q3W | 379 | LTFU |
| DAR | int-1 | 61 | Q3W | 64 | DB |
| DAR | low | 247 | Q3W | 146 | DB |
| DAR | int-1 | 85 | Q3W | 195 | LTFU |
| DAR | int-1 | 76 | Q3W | 254 | OL |
| DAR (pt had response from wks 37–45) | int-1 | 80 | Q2W | 489 | OL |
| DAR | int-1 | 191 | Q2W | 788 | LTFU |
| DAR (pt had response from wks 10–37) | low | 296 | Q2W | 743 | LTFU |

Conclusion
In this study, there were no differences in rates of death (mostly reported in LTFU) or AML between DAR and PBO arms. Pts with int-1 risk MDS had worse prognosis. Findings are limited because the study was not powered to detect differences in survival and pts could receive any therapy of physician’s choice in LTFU. In conclusion, DAR is a safe treatment and lowered transfusion rates associated with anemia of low/int-1 risk MDS.
Session topic: 10. Myelodysplastic syndromes – Clinical
Keyword(s): Anemia, Darbepoetin alfa, MDS


