
Contributions
Abstract: S853
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 16:15 - 16:30
Location: Victoria Hall
Background
Bruton tyrosine kinase (BTK) is a clinically validated target in Waldenström Macroglobulinemia (WM). Acalabrutinib is a highly selective, potent, covalent BTK inhibitor.
Aims
The efficacy and safety of acalabrutinib was evaluated in a Phase 2 study of patients with treatment-naive (TN) or relapsed/refractory (R/R) WM.
Methods
Patients with TN or R/R WM received 100 mg acalabrutinib BID (or 200 mg QD [n=6], later switched to 100 mg BID) in 28-day cycles until progressive disease (PD) or intolerance. The primary endpoint was investigator-assessed overall response rate (ORR). Secondary endpoints included duration of response (DOR), progression-free survival (PFS), overall survival (OS), safety and pharmacokinetics (PK).
Results
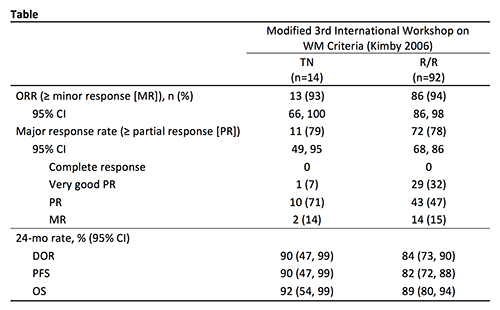
One hundred six patients (14 TN and 92 R/R) were treated. In all patients, the median age was 69 years (range 39-90), 94% had ECOG PS ≤1, and median serum IgM level was 3615 mg/dL (range 291-9740). R/R patients had a median of 2 prior therapies (range 1-7). At a 25-mo median follow-up, 7 (50%) TN patients and 70 (76%) R/R patients remain on treatment. Discontinuations were primarily due to PD (TN: 0 patients; R/R: 9 patients), adverse events (AEs; TN: 3 patients; R/R: 3 patients), and investigator decision (TN: 2 patients; R/R: 4 patients). BTK occupancy and PK parameters were consistent with previous acalabrutinib studies. Efficacy outcomes are listed in the Table. The most common AEs of any grade were headache (39%), diarrhea (31%), contusion (29%), and dizziness (25%). The most common Grade 3/4 AEs were neutropenia (16%), pneumonia (7%), anemia, increased alanine aminotransferase, and hyponatremia (each 5%). Atrial fibrillation occurred in 3 patients; 1 case was Grade 3. Bleeding events occurred in 57% of patients, the most common of which were contusion (29%) and epistaxis (13%). Four bleeding events were Grade 3/4: epistaxis, hematuria, dysfunctional uterine bleeding, and retinal hemorrhage. There were 5 Grade 5 events: pneumonia, glioblastoma multiforme, esophageal carcinoma, myocardial ischemia, and intracranial hematoma.
Conclusion
Acalabrutinib is a highly effective treatment for WM with durable responses and limited toxicity.
Session topic: 20. Indolent Non-Hodgkin lymphoma – Clinical
Keyword(s): Clinical Trial, Kinase Inhibitor, Targeted therapy, Waldenstrom's macroglobulinemia
Abstract: S853
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 16:15 - 16:30
Location: Victoria Hall
Background
Bruton tyrosine kinase (BTK) is a clinically validated target in Waldenström Macroglobulinemia (WM). Acalabrutinib is a highly selective, potent, covalent BTK inhibitor.
Aims
The efficacy and safety of acalabrutinib was evaluated in a Phase 2 study of patients with treatment-naive (TN) or relapsed/refractory (R/R) WM.
Methods
Patients with TN or R/R WM received 100 mg acalabrutinib BID (or 200 mg QD [n=6], later switched to 100 mg BID) in 28-day cycles until progressive disease (PD) or intolerance. The primary endpoint was investigator-assessed overall response rate (ORR). Secondary endpoints included duration of response (DOR), progression-free survival (PFS), overall survival (OS), safety and pharmacokinetics (PK).
Results
One hundred six patients (14 TN and 92 R/R) were treated. In all patients, the median age was 69 years (range 39-90), 94% had ECOG PS ≤1, and median serum IgM level was 3615 mg/dL (range 291-9740). R/R patients had a median of 2 prior therapies (range 1-7). At a 25-mo median follow-up, 7 (50%) TN patients and 70 (76%) R/R patients remain on treatment. Discontinuations were primarily due to PD (TN: 0 patients; R/R: 9 patients), adverse events (AEs; TN: 3 patients; R/R: 3 patients), and investigator decision (TN: 2 patients; R/R: 4 patients). BTK occupancy and PK parameters were consistent with previous acalabrutinib studies. Efficacy outcomes are listed in the Table. The most common AEs of any grade were headache (39%), diarrhea (31%), contusion (29%), and dizziness (25%). The most common Grade 3/4 AEs were neutropenia (16%), pneumonia (7%), anemia, increased alanine aminotransferase, and hyponatremia (each 5%). Atrial fibrillation occurred in 3 patients; 1 case was Grade 3. Bleeding events occurred in 57% of patients, the most common of which were contusion (29%) and epistaxis (13%). Four bleeding events were Grade 3/4: epistaxis, hematuria, dysfunctional uterine bleeding, and retinal hemorrhage. There were 5 Grade 5 events: pneumonia, glioblastoma multiforme, esophageal carcinoma, myocardial ischemia, and intracranial hematoma.

Conclusion
Acalabrutinib is a highly effective treatment for WM with durable responses and limited toxicity.
Session topic: 20. Indolent Non-Hodgkin lymphoma – Clinical
Keyword(s): Clinical Trial, Kinase Inhibitor, Targeted therapy, Waldenstrom's macroglobulinemia


