
Contributions
Abstract: S847
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 16:00 - 16:15
Location: Room A1
Background
Despite recent therapeutic advances, multiple myeloma (MM) remains an incurable plasma cell malignancy. Pomalidomide (POM) is a standard-of-care treatment in relapsed or refractory MM (RRMM) and has demonstrated synergistic anti-myeloma activity with dexamethasone (DEX) and proteasome inhibitors, as well as cytotoxic effects in lenalidomide (LEN)-resistant cells. POM + DEX is approved in the European Union for patients with RRMM who received ≥ 2 prior therapies, including LEN and bortezomib (BORT), and has been investigated after LEN-based treatment. As LEN becomes increasingly established in up-front treatment of MM, patients who have exhausted the benefit of LEN represent a clinically relevant unmet medical need. Here we report final progression-free survival (PFS) and safety data from a phase 3 triplet trial in an entirely post–LEN-treated population, comparing POM, BORT, and low-dose DEX (PVd) vs BORT and low-dose DEX (Vd) in patients who received 1 to 3 prior therapies.
Aims
To compare the efficacy and safety of PVd vs Vd in LEN-exposed patients with RRMM.
Methods
Key eligibility criteria included 1 to 3 prior regimens and ≥ 2 cycles of prior LEN therapy. Patients were randomized 1:1 to receive PVd or Vd treatment in 21-day cycles: POM 4 mg/day on days 1-14 (PVd arm only); BORT 1.3 mg/m2 on days 1, 4, 8, and 11 of cycles 1-8 and on days 1 and 8 of cycle 9 and beyond; and DEX 20 mg/day (10 mg/day if aged > 75 years) on the days of and after BORT. The primary endpoint was PFS. All pts provided informed consent.
Results
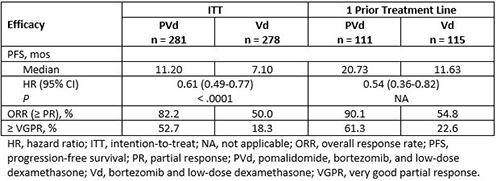
A total of 559 patients were enrolled: 281 patients in the PVd arm and 278 in the Vd arm. Demographic, baseline, and prior disease characteristics were generally well balanced between the 2 treatment arms. Median age was 67 and 68 years, respectively. All patients had prior LEN (71% vs 69% were LEN refractory), 72% vs 73% had prior BORT, and 70% vs 66% were refractory to their last treatment. Median number of prior lines of therapy was 2; 40% and 41% had 1 prior line of treatment in the PVd and Vd arms, respectively. Median follow-up was 16 months. Compared with Vd, PVd treatment significantly reduced the risk of progression or death by 39% and resulted in deeper responses in the intention-to-treat (ITT) population (Table). Median PFS was 11.20 vs 7.10 months in the ITT population and 20.73 vs 11.63 months in patients with only 1 prior treatment including LEN. Data for the overall survival analysis are not yet mature. Neutropenia (42% vs 9%), infections (31% vs 18%), and thrombocytopenia (27% vs 29%) were among the most frequently reported grade 3/4 treatment-emergent adverse events.
Conclusion
The OPTIMISMM phase 3 study in early RRMM reported a significant and clinically meaningful PFS improvement in patients who were entirely LEN exposed, including 70% of patients who were refractory to LEN. Of note, the PFS and overall response rate results showed improved benefit with PVd over Vd in patients who had only 1 prior line of treatment. Follow-up for long-term survival is ongoing. The safety of POM-based treatment was manageable and consistent with its well-established profile.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Malignancy, Multiple Myeloma, Refractory, Safety
Abstract: S847
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 16:00 - 16:15
Location: Room A1
Background
Despite recent therapeutic advances, multiple myeloma (MM) remains an incurable plasma cell malignancy. Pomalidomide (POM) is a standard-of-care treatment in relapsed or refractory MM (RRMM) and has demonstrated synergistic anti-myeloma activity with dexamethasone (DEX) and proteasome inhibitors, as well as cytotoxic effects in lenalidomide (LEN)-resistant cells. POM + DEX is approved in the European Union for patients with RRMM who received ≥ 2 prior therapies, including LEN and bortezomib (BORT), and has been investigated after LEN-based treatment. As LEN becomes increasingly established in up-front treatment of MM, patients who have exhausted the benefit of LEN represent a clinically relevant unmet medical need. Here we report final progression-free survival (PFS) and safety data from a phase 3 triplet trial in an entirely post–LEN-treated population, comparing POM, BORT, and low-dose DEX (PVd) vs BORT and low-dose DEX (Vd) in patients who received 1 to 3 prior therapies.
Aims
To compare the efficacy and safety of PVd vs Vd in LEN-exposed patients with RRMM.
Methods
Key eligibility criteria included 1 to 3 prior regimens and ≥ 2 cycles of prior LEN therapy. Patients were randomized 1:1 to receive PVd or Vd treatment in 21-day cycles: POM 4 mg/day on days 1-14 (PVd arm only); BORT 1.3 mg/m2 on days 1, 4, 8, and 11 of cycles 1-8 and on days 1 and 8 of cycle 9 and beyond; and DEX 20 mg/day (10 mg/day if aged > 75 years) on the days of and after BORT. The primary endpoint was PFS. All pts provided informed consent.
Results
A total of 559 patients were enrolled: 281 patients in the PVd arm and 278 in the Vd arm. Demographic, baseline, and prior disease characteristics were generally well balanced between the 2 treatment arms. Median age was 67 and 68 years, respectively. All patients had prior LEN (71% vs 69% were LEN refractory), 72% vs 73% had prior BORT, and 70% vs 66% were refractory to their last treatment. Median number of prior lines of therapy was 2; 40% and 41% had 1 prior line of treatment in the PVd and Vd arms, respectively. Median follow-up was 16 months. Compared with Vd, PVd treatment significantly reduced the risk of progression or death by 39% and resulted in deeper responses in the intention-to-treat (ITT) population (Table). Median PFS was 11.20 vs 7.10 months in the ITT population and 20.73 vs 11.63 months in patients with only 1 prior treatment including LEN. Data for the overall survival analysis are not yet mature. Neutropenia (42% vs 9%), infections (31% vs 18%), and thrombocytopenia (27% vs 29%) were among the most frequently reported grade 3/4 treatment-emergent adverse events.

Conclusion
The OPTIMISMM phase 3 study in early RRMM reported a significant and clinically meaningful PFS improvement in patients who were entirely LEN exposed, including 70% of patients who were refractory to LEN. Of note, the PFS and overall response rate results showed improved benefit with PVd over Vd in patients who had only 1 prior line of treatment. Follow-up for long-term survival is ongoing. The safety of POM-based treatment was manageable and consistent with its well-established profile.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Malignancy, Multiple Myeloma, Refractory, Safety


