
Contributions
Abstract: S839
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 12:00 - 12:15
Location: Room A9
Background
Andexanet alfa (andexanet) is a recombinant protein that acts as a factor Xa (FXa) decoy to bind and sequester FXa inhibitors (apixaban, rivaroxaban, edoxaban, and betrixaban). A naïve-pooled pharmacokinetic (PK)/pharmacodynamics (PD) model, developed in Phase 2 studies in healthy subjects, predicted the andexanet regimen required to reverse anticoagulation by FXa inhibitors. Preliminary analysis of the ongoing Phase 3b/4 study (ANNEXA-4) demonstrated that the naïve-pooled model was predictive of the anti-FXa activity reversal in patients with acute major bleeding who were anticoagulated with FXa inhibitors. It is not known whether patient characteristics (e.g., impaired renal function, advanced age) may alter PK and affect the accuracy of the model.
Aims
The first interim data from the ANNEXA-4 study in patients with acute major bleeding was compared to predictions from the naïve-pooled PK/PD model. Additionally, enhanced analyses of the model that include evaluation of intrinsic factors that might affect both FXa inhibitor and andexanet levels in this patient population are ongoing.
Methods
In ANNEXA-4, an ongoing prospective, open-label study, bleeding patients anticoagulated with a FXa inhibitor received IV andexanet bolus (400 or 800 mg) followed by 120-min infusion (4 or 8 mg/min). Anti-FXa activity was measured before andexanet administration (baseline), at end of bolus (EOB), end of infusion, and 4, 8, and 12 h after infusion. The relationship between baseline anti-FXa activity and reversal in healthy subjects was used to develop the naïve-pooled PK/PD model and then to predict the percent reversal of anti-FXa activity for patients with acute major bleeding. Refinement of the PK/PD model includes assessment of intrinsic factors such as renal function, age and body weight on both FXa and andexanet exposure.
Results
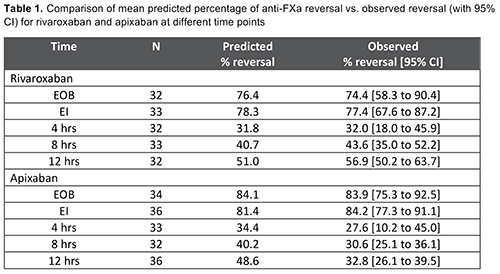
In the first interim analysis of ANNEXA-4, 73 patients (apixaban, 39; rivaroxaban, 34) had plasma levels available for model qualification. The mean observed percent reversal of anti-FXa activity for rivaroxaban and apixaban was well predicted by the healthy subject PK/PD model; the point estimates fell within the 90% confidence intervals of predicted values (Table 1). The predicted reversal fit closely the observed confidence intervals through the first 4 h for rivaroxaban and apixaban; it extended through all evaluated time points for rivaroxaban but only through the post 4-h time points for apixaban, possibly due to higher baseline anti-FXa activity levels seen in some apixaban patients. The revised PK/PD model identified body weight as a significant covariate for andexanet exposure, and incorporated published covariates for rivaroxaban and apixaban, including renal function, age and lean body mass. This revised model is being used in all subsequent PK/PD analysis in samples from bleeding patients.
Conclusion
The naïve-pooled PK/PD model in healthy subjects closely predicted the percent reversal of anti-FXa activity by andexanet in patients receiving apixaban or rivaroxaban who presented with acute major bleeding. At later time points, the observed apixaban anti-FXa activity reversal did not overlap with that predicted by the naïve-pooled model. Incorporation of intrinsic factors (renal function and age) into the PK/PD model may provide more robust prediction of anti-FXa activity reversal in patients initially seen as outliers.
Session topic: 35. Thrombosis and vascular biology & translational Research
Keyword(s): Anticoagulation, Factor Xa, Pharmacokinetic
Abstract: S839
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 12:00 - 12:15
Location: Room A9
Background
Andexanet alfa (andexanet) is a recombinant protein that acts as a factor Xa (FXa) decoy to bind and sequester FXa inhibitors (apixaban, rivaroxaban, edoxaban, and betrixaban). A naïve-pooled pharmacokinetic (PK)/pharmacodynamics (PD) model, developed in Phase 2 studies in healthy subjects, predicted the andexanet regimen required to reverse anticoagulation by FXa inhibitors. Preliminary analysis of the ongoing Phase 3b/4 study (ANNEXA-4) demonstrated that the naïve-pooled model was predictive of the anti-FXa activity reversal in patients with acute major bleeding who were anticoagulated with FXa inhibitors. It is not known whether patient characteristics (e.g., impaired renal function, advanced age) may alter PK and affect the accuracy of the model.
Aims
The first interim data from the ANNEXA-4 study in patients with acute major bleeding was compared to predictions from the naïve-pooled PK/PD model. Additionally, enhanced analyses of the model that include evaluation of intrinsic factors that might affect both FXa inhibitor and andexanet levels in this patient population are ongoing.
Methods
In ANNEXA-4, an ongoing prospective, open-label study, bleeding patients anticoagulated with a FXa inhibitor received IV andexanet bolus (400 or 800 mg) followed by 120-min infusion (4 or 8 mg/min). Anti-FXa activity was measured before andexanet administration (baseline), at end of bolus (EOB), end of infusion, and 4, 8, and 12 h after infusion. The relationship between baseline anti-FXa activity and reversal in healthy subjects was used to develop the naïve-pooled PK/PD model and then to predict the percent reversal of anti-FXa activity for patients with acute major bleeding. Refinement of the PK/PD model includes assessment of intrinsic factors such as renal function, age and body weight on both FXa and andexanet exposure.
Results
In the first interim analysis of ANNEXA-4, 73 patients (apixaban, 39; rivaroxaban, 34) had plasma levels available for model qualification. The mean observed percent reversal of anti-FXa activity for rivaroxaban and apixaban was well predicted by the healthy subject PK/PD model; the point estimates fell within the 90% confidence intervals of predicted values (Table 1). The predicted reversal fit closely the observed confidence intervals through the first 4 h for rivaroxaban and apixaban; it extended through all evaluated time points for rivaroxaban but only through the post 4-h time points for apixaban, possibly due to higher baseline anti-FXa activity levels seen in some apixaban patients. The revised PK/PD model identified body weight as a significant covariate for andexanet exposure, and incorporated published covariates for rivaroxaban and apixaban, including renal function, age and lean body mass. This revised model is being used in all subsequent PK/PD analysis in samples from bleeding patients.

Conclusion
The naïve-pooled PK/PD model in healthy subjects closely predicted the percent reversal of anti-FXa activity by andexanet in patients receiving apixaban or rivaroxaban who presented with acute major bleeding. At later time points, the observed apixaban anti-FXa activity reversal did not overlap with that predicted by the naïve-pooled model. Incorporation of intrinsic factors (renal function and age) into the PK/PD model may provide more robust prediction of anti-FXa activity reversal in patients initially seen as outliers.
Session topic: 35. Thrombosis and vascular biology & translational Research
Keyword(s): Anticoagulation, Factor Xa, Pharmacokinetic


