
Contributions
Abstract: S803
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 12:30 - 12:45
Location: Room A1
Background
Rituximab (R) plus CHOP (R-CHOP) is standard of care for pts with previously untreated DLBCL. Although most pts have long-term responses, up to 40% relapse or fail to achieve a remission. Atezolizumab (atezo) is a fully humanised anti-programmed death-ligand 1 (PD-L1) antibody with a complementary mechanism of action to R. An ongoing phase I/II study (NCT02596971) is evaluating the safety and efficacy of atezo in combination with R-CHOP (R-CHOP-atezo) in DLBCL pts. We report interim data.
Aims
To assess the safety and preliminary efficacy of R-CHOP-atezo in previously untreated pts with DLBCL.
Methods
Pts (aged ≥18 years, ECOG PS 0–2) with advanced DLBCL (Ann Arbor stage III/IV, International Prognostic Index [IPI] score ≥2 or stage II with bulky disease [at least one lesion ≥7cm]) received 8 cycles (each 21 days) of induction treatment with R-CHOP-atezo (R 375 mg/m2 IV on Day 1 [Cycles 1–8], atezo 1200 mg IV on Day 1 [Cycles 2−8], CHOP [6 or 8 cycles as determined by the investigator (INV)]). Pts who achieved a CR at end of induction (EOI) received consolidation treatment with atezo 1200 mg IV on Day 1 of Cycles 9–25, every 21 days for 12 months. The primary endpoints were safety, and efficacy as determined by CR rate at EOI by independent review committee (IRC) using modified Lugano 2014 criteria. Secondary endpoints included CR rate at EOI assessed by INV using modified Lugano 2014, and by IRC and INV using Cheson 2007. An interim analysis was pre-planned after 15 consecutive pts had completed the EOI response assessment. The data cut-off was 20 November 2017.
Results
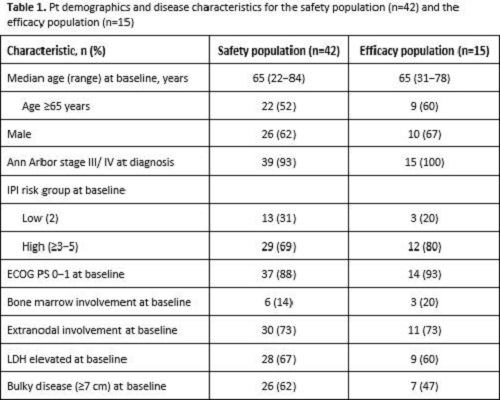
In total, 42 pts were enrolled and received treatment (safety population). At the time of data cut-off, 20 pts were receiving ongoing induction treatment, 5 had discontinued (3 AEs, 1 protocol violation, 1 withdrawal) and 17 had completed induction, of whom 14 had entered consolidation. Median (range) observation time was 5.3 (0.7−7.2) months for induction and only 1.6 (0.7−5.1) months for consolidation. Therefore, this report focuses on preliminary data from the safety population during induction only. Pt demographics and disease characteristics are shown in Table 1. Among 15 pts evaluable for response (efficacy population), 13 (87%) achieved a CR, and 2 (13%) had PD according to IRC and INV using modified Lugano 2014. Response assessment using Cheson 2007 showed 11 (73%) CRs, 2 (13%) PRs and 2 (13%) pts with PD, consistent between INV and IRC review. Preliminary safety data from induction therapy showed that all pts had ≥1 AE and 27/42 pts (64%) had a grade (gr) 3−4 AE. Regardless of causality, the most common gr 3–4 AEs were neutropenia (16 pts [38%], 6 received G-CSF prophylaxis) and febrile neutropenia (4 pts [9.5%], 3 received G-CSF prophylaxis). No deaths were reported. Serious AEs were reported in 13 pts (31%). AEs led to dose reduction in 8 pts (19%) (gr 4 neutropenia, gr 3 pancytopenia, gr 1−2 peripheral neuropathy) and withdrawal of any component in 3 pts (7%) (gr 3 neutropenia, gr 3 transaminase and asymptomatic lipase increases, gr 2 hyperthyroidism). Three pts had atezo-related AEs (gr 3 lipase and transaminase increases).
Conclusion
Interim data demonstrate that first-line R-CHOP-atezo shows encouraging efficacy and acceptable toxicity in untreated pts with DLBCL. Updated results, including minimal residual disease and cell of origin data, will be presented.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): DLBCL, Immunotherapy, Monoclonal antibody, Targeted therapy
Abstract: S803
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 12:30 - 12:45
Location: Room A1
Background
Rituximab (R) plus CHOP (R-CHOP) is standard of care for pts with previously untreated DLBCL. Although most pts have long-term responses, up to 40% relapse or fail to achieve a remission. Atezolizumab (atezo) is a fully humanised anti-programmed death-ligand 1 (PD-L1) antibody with a complementary mechanism of action to R. An ongoing phase I/II study (NCT02596971) is evaluating the safety and efficacy of atezo in combination with R-CHOP (R-CHOP-atezo) in DLBCL pts. We report interim data.
Aims
To assess the safety and preliminary efficacy of R-CHOP-atezo in previously untreated pts with DLBCL.
Methods
Pts (aged ≥18 years, ECOG PS 0–2) with advanced DLBCL (Ann Arbor stage III/IV, International Prognostic Index [IPI] score ≥2 or stage II with bulky disease [at least one lesion ≥7cm]) received 8 cycles (each 21 days) of induction treatment with R-CHOP-atezo (R 375 mg/m2 IV on Day 1 [Cycles 1–8], atezo 1200 mg IV on Day 1 [Cycles 2−8], CHOP [6 or 8 cycles as determined by the investigator (INV)]). Pts who achieved a CR at end of induction (EOI) received consolidation treatment with atezo 1200 mg IV on Day 1 of Cycles 9–25, every 21 days for 12 months. The primary endpoints were safety, and efficacy as determined by CR rate at EOI by independent review committee (IRC) using modified Lugano 2014 criteria. Secondary endpoints included CR rate at EOI assessed by INV using modified Lugano 2014, and by IRC and INV using Cheson 2007. An interim analysis was pre-planned after 15 consecutive pts had completed the EOI response assessment. The data cut-off was 20 November 2017.
Results
In total, 42 pts were enrolled and received treatment (safety population). At the time of data cut-off, 20 pts were receiving ongoing induction treatment, 5 had discontinued (3 AEs, 1 protocol violation, 1 withdrawal) and 17 had completed induction, of whom 14 had entered consolidation. Median (range) observation time was 5.3 (0.7−7.2) months for induction and only 1.6 (0.7−5.1) months for consolidation. Therefore, this report focuses on preliminary data from the safety population during induction only. Pt demographics and disease characteristics are shown in Table 1. Among 15 pts evaluable for response (efficacy population), 13 (87%) achieved a CR, and 2 (13%) had PD according to IRC and INV using modified Lugano 2014. Response assessment using Cheson 2007 showed 11 (73%) CRs, 2 (13%) PRs and 2 (13%) pts with PD, consistent between INV and IRC review. Preliminary safety data from induction therapy showed that all pts had ≥1 AE and 27/42 pts (64%) had a grade (gr) 3−4 AE. Regardless of causality, the most common gr 3–4 AEs were neutropenia (16 pts [38%], 6 received G-CSF prophylaxis) and febrile neutropenia (4 pts [9.5%], 3 received G-CSF prophylaxis). No deaths were reported. Serious AEs were reported in 13 pts (31%). AEs led to dose reduction in 8 pts (19%) (gr 4 neutropenia, gr 3 pancytopenia, gr 1−2 peripheral neuropathy) and withdrawal of any component in 3 pts (7%) (gr 3 neutropenia, gr 3 transaminase and asymptomatic lipase increases, gr 2 hyperthyroidism). Three pts had atezo-related AEs (gr 3 lipase and transaminase increases).

Conclusion
Interim data demonstrate that first-line R-CHOP-atezo shows encouraging efficacy and acceptable toxicity in untreated pts with DLBCL. Updated results, including minimal residual disease and cell of origin data, will be presented.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): DLBCL, Immunotherapy, Monoclonal antibody, Targeted therapy


