
Contributions
Abstract: S118
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:15 - 12:30
Location: Room A4
Background
Spleen tyrosine kinase (SYK) is a non-receptor tyrosine kinase primarily expressed in hematopoietic cells. Constitutive activation of SYK in acute myeloid leukemia (AML) has been reported; targeted inhibition of SYK-induced differentiation in vitro demonstrated anti-leukemia activity in AML mouse models. SYK promotes leukemogenesis by directly phosphorylating the FLT3 receptor, and inducing MEIS1 in conjunction with HOXA9 to form a regulatory loop in KMT2A (mixed lineage leukemia [MLL]) rearranged leukemia. Entospletinib (ENTO) is an orally bioavailable, selective inhibitor of SYK with activity in myeloid and B-lymphoid malignancies.
Aims
Evaluate the efficacy of ENTO in untreated AML patients (pts) as monotherapy followed by ENTO plus standard 7+3 induction chemotherapy (cytarabine 100 mg/m2/d, days 1-7 plus daunorubicin 60 mg/m2/d, days 1-3).
Methods
In this phase 1b/2 study (NCT02343939), pts aged 18 to 70 years with previously untreated AML, preserved organ function, and ECOG ≤2 were eligible to receive ENTO 400 mg BID for 14 days as monotherapy lead-in (days -14 to 0) followed by combination with standard 7+3 induction chemotherapy (ENTO+7+3) for a maximum of two induction cycles. Response assessments were made per the modified International Working Group criteria.
Results
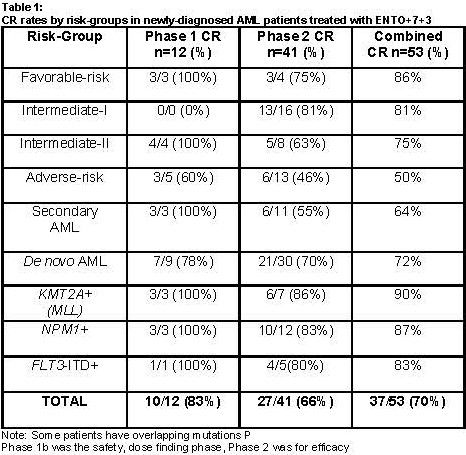
Fifty-three pts (n=12 in Phase 1b and n=41 Phase 2) with de novo and secondary AML were enrolled with a median age of 60 (range, 18-78) years. 58% pts were male and per the 2010 European LeukemiaNet guidelines, there were 7 (13%), 16 (30%), 12 (27%) and 18 (34%) pts in the favorable, intermediate I, intermediate II, and adverse risk groups, respectively. ENTO monotherapy and ENTO+7+3 were well tolerated. Most of the adverse events (AEs) that occurred in the ENTO+7+3 phase were consistent with those expected from 7+3 induction chemotherapy. Seven (13%) pts had a grade ≥3 rash and 8 (15%) pts had grade ≥3 transaminitis or hyperbilirubinemia. Overall, 16 (30%) pts needed ENTO dose interruptions or reduction due to AEs, and 8 (15%) pts discontinued study drug due to AEs. Forty-one (77%) pts had grade ≥3 febrile neutropenia. There were no deaths with 30-day induction mortality of 0%. With the 14-day lead-in one pt with t(11;19) translocation achieved a CR with ENTO monotherapy alone, prior to the combination phase. Of note, 15 (28%) pts did not get the full 14-day lead-in due to pt or physician preference, and 12 (23%) pts required concomitant hydroxyurea due to rising white blood cell counts. The CR rate with ENTO+7+3 was 70%. Fourteen (26%) pts had secondary AML and the CR rate in this group was 64%. In addition, higher than historical control CR rates were noted in pts with NPM1 mutation (n=15, CR 87%), FLT3-ITD (n=6, CR 83%) and KMT2A gene rearrangements (n=10, CR 90%). After a median follow-up (f/u) of 8 months (m), the median event-free survival, relapse-free survival and overall survival were 7m, 7m, and not reached, respectively. Fifteen (28%) pts received allogeneic stem cell transplantation in the post-remission setting.
Conclusion
In untreated AML patients, ENTO+7+3 is safe and demonstrates high remission rates in specific molecular subgroups. In our study, pts with secondary AML, NPM1, FLT3-ITD mutations, and KMT2A gene rearrangements were noted to have higher CR rates than historical controls. Durability of response with longer f/u is pending and correlative biomarker studies evaluating HOXA9/MEIS1 in pts with NPM1, FLT3-ITD mutated and KMT2A rearranged leukemia treated with ENTO are being presented separately.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Acute Myeloid Leukemia, Induction, Tyrosine kinase inhibitor
Abstract: S118
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:15 - 12:30
Location: Room A4
Background
Spleen tyrosine kinase (SYK) is a non-receptor tyrosine kinase primarily expressed in hematopoietic cells. Constitutive activation of SYK in acute myeloid leukemia (AML) has been reported; targeted inhibition of SYK-induced differentiation in vitro demonstrated anti-leukemia activity in AML mouse models. SYK promotes leukemogenesis by directly phosphorylating the FLT3 receptor, and inducing MEIS1 in conjunction with HOXA9 to form a regulatory loop in KMT2A (mixed lineage leukemia [MLL]) rearranged leukemia. Entospletinib (ENTO) is an orally bioavailable, selective inhibitor of SYK with activity in myeloid and B-lymphoid malignancies.
Aims
Evaluate the efficacy of ENTO in untreated AML patients (pts) as monotherapy followed by ENTO plus standard 7+3 induction chemotherapy (cytarabine 100 mg/m2/d, days 1-7 plus daunorubicin 60 mg/m2/d, days 1-3).
Methods
In this phase 1b/2 study (NCT02343939), pts aged 18 to 70 years with previously untreated AML, preserved organ function, and ECOG ≤2 were eligible to receive ENTO 400 mg BID for 14 days as monotherapy lead-in (days -14 to 0) followed by combination with standard 7+3 induction chemotherapy (ENTO+7+3) for a maximum of two induction cycles. Response assessments were made per the modified International Working Group criteria.
Results
Fifty-three pts (n=12 in Phase 1b and n=41 Phase 2) with de novo and secondary AML were enrolled with a median age of 60 (range, 18-78) years. 58% pts were male and per the 2010 European LeukemiaNet guidelines, there were 7 (13%), 16 (30%), 12 (27%) and 18 (34%) pts in the favorable, intermediate I, intermediate II, and adverse risk groups, respectively. ENTO monotherapy and ENTO+7+3 were well tolerated. Most of the adverse events (AEs) that occurred in the ENTO+7+3 phase were consistent with those expected from 7+3 induction chemotherapy. Seven (13%) pts had a grade ≥3 rash and 8 (15%) pts had grade ≥3 transaminitis or hyperbilirubinemia. Overall, 16 (30%) pts needed ENTO dose interruptions or reduction due to AEs, and 8 (15%) pts discontinued study drug due to AEs. Forty-one (77%) pts had grade ≥3 febrile neutropenia. There were no deaths with 30-day induction mortality of 0%. With the 14-day lead-in one pt with t(11;19) translocation achieved a CR with ENTO monotherapy alone, prior to the combination phase. Of note, 15 (28%) pts did not get the full 14-day lead-in due to pt or physician preference, and 12 (23%) pts required concomitant hydroxyurea due to rising white blood cell counts. The CR rate with ENTO+7+3 was 70%. Fourteen (26%) pts had secondary AML and the CR rate in this group was 64%. In addition, higher than historical control CR rates were noted in pts with NPM1 mutation (n=15, CR 87%), FLT3-ITD (n=6, CR 83%) and KMT2A gene rearrangements (n=10, CR 90%). After a median follow-up (f/u) of 8 months (m), the median event-free survival, relapse-free survival and overall survival were 7m, 7m, and not reached, respectively. Fifteen (28%) pts received allogeneic stem cell transplantation in the post-remission setting.

Conclusion
In untreated AML patients, ENTO+7+3 is safe and demonstrates high remission rates in specific molecular subgroups. In our study, pts with secondary AML, NPM1, FLT3-ITD mutations, and KMT2A gene rearrangements were noted to have higher CR rates than historical controls. Durability of response with longer f/u is pending and correlative biomarker studies evaluating HOXA9/MEIS1 in pts with NPM1, FLT3-ITD mutated and KMT2A rearranged leukemia treated with ENTO are being presented separately.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Acute Myeloid Leukemia, Induction, Tyrosine kinase inhibitor


