
Contributions
Abstract: S117
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:00 - 12:15
Location: Room A4
Background
Among patients over the age of 60, a considerable number of patients with Acute Myeloid Leukaemia (AML) are not considered for conventional induction chemotherapy, so survival is poor, with only approximately 10% of patients surviving beyond 2 years when treated with standard of care (demethylation agents or low dose ara-C (LDAC)). In the pivotal trials demethylation agents improve median survival, but not overall survival. Therefore there remains a significant unmet need in this patient group. Tosedostat is a selective, oral aminopeptidase inhibitor. Since Phase I/II trials of tosedostat as monotherapy showed acceptable toxicity and potential activity in relapsed AML it was included, combined with LDAC, as an option in the LI-1 “pick-a-winner” trial.
Aims
To assess the efficacy of LDAC+tosedostat versus LDAC alone in patients aged 60+ unsuitable for intensive therapy in a “pick-a-winner” design. This design allows several treatments to be assessed simultaneously compared with LDAC in a randomised fashion, with the aim of doubling 2-year survival from 11% to 22% (HR 0.70). There are two interim assessments: after 50 patients per arm are recruited, remission rates must improve by ≥2.5%; the second interim analysis occurs after 170 deaths are seen, when the hazard ratio must be <0.85.
Methods
Tosedostat was given orally at 120mg once a day for up to 6 months. LDAC was given at 20mg bd subcutaneously on days 1-10 of each course, with courses of LDAC occurring at 4-6 wk intervals. To enter the randomisation patients needed to fulfil specific cardiac entry criteria. Toxicities were recorded using NCI-CTC version 3. At the second interim analysis after 183 events tosedostat failed to pass the second assessment, and the arm was therefore closed. Results here are based upon a median follow-up of 18.9 months.
Results
Between 6/2014 and 2/2017, 245 patients, median age 76 years (range 60-88) entered the randomisation. Overall 60% were male; 66% had de Novo AML, 28% secondary AML, and 6% high risk MDS; 1% had favourable, 73% intermediate and 26% adverse cytogenetics. By validated Wheatley index, 2% were good risk, 36% standard risk and 63% poor risk. A median of 2 courses was delivered in either arm (mean 2.9 LDAC+tosedostat vs 2.3 LDAC).
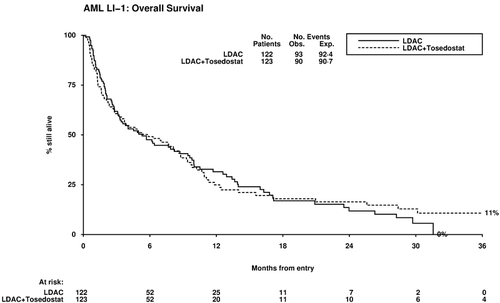
Overall, complete remission was achieved in 18% of patients (LDAC+tosedostat 22%, LDAC 14%, OR 0.59 (0.31-1.13) p=0.11). Thirty-day mortality was not significantly increased (17% vs 13%, HR 1.44 (0.75-2.78) p=0.3); but overall survival showed no difference (2-year OS 16% vs 12%, HR 0.99 (0.74-1.32) p=0.9). Causes of death were: resistant/recurrent disease 39 vs 61; infection 20 vs 10; haemorrhage 8 vs 0; cardiac 5 vs 3; multiple 13 vs 8; other/unknown 5 vs 11. Relapse-free survival did not significantly differ (HR 0.93 (0.41-2.16) p=0.9) with median OS 28.4m vs 24.0m in responders (p=0.6); non-remitters had median OS 2.8m vs 3.3m (HR 1.20 (0.88-1.63) p=0.2). Stratified analyses failed to identify any subgroup of patients benefitting from tosedostat. Although rates of grade 3+ toxicity were low, tosedostat was associated with diarrhoea, increased cardiac toxicity, and increade use of platelets (mean 5.0 vs 3.5 p=0.006).
Conclusion
Despite promising early data and acceptable tolerability, we did not find evidence that the addition of tosedostat to LDAC produced a survival benefit in this group of patients, with the anticipated hazard ratio of 0.70 being outside the 95% confidence intervals at second interim analysis. Acknowledgements: We are grateful to CTI Biopharma for providing drug and support for this Investigator Initiated Study.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): AML, Clinical Trial
Abstract: S117
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:00 - 12:15
Location: Room A4
Background
Among patients over the age of 60, a considerable number of patients with Acute Myeloid Leukaemia (AML) are not considered for conventional induction chemotherapy, so survival is poor, with only approximately 10% of patients surviving beyond 2 years when treated with standard of care (demethylation agents or low dose ara-C (LDAC)). In the pivotal trials demethylation agents improve median survival, but not overall survival. Therefore there remains a significant unmet need in this patient group. Tosedostat is a selective, oral aminopeptidase inhibitor. Since Phase I/II trials of tosedostat as monotherapy showed acceptable toxicity and potential activity in relapsed AML it was included, combined with LDAC, as an option in the LI-1 “pick-a-winner” trial.
Aims
To assess the efficacy of LDAC+tosedostat versus LDAC alone in patients aged 60+ unsuitable for intensive therapy in a “pick-a-winner” design. This design allows several treatments to be assessed simultaneously compared with LDAC in a randomised fashion, with the aim of doubling 2-year survival from 11% to 22% (HR 0.70). There are two interim assessments: after 50 patients per arm are recruited, remission rates must improve by ≥2.5%; the second interim analysis occurs after 170 deaths are seen, when the hazard ratio must be <0.85.
Methods
Tosedostat was given orally at 120mg once a day for up to 6 months. LDAC was given at 20mg bd subcutaneously on days 1-10 of each course, with courses of LDAC occurring at 4-6 wk intervals. To enter the randomisation patients needed to fulfil specific cardiac entry criteria. Toxicities were recorded using NCI-CTC version 3. At the second interim analysis after 183 events tosedostat failed to pass the second assessment, and the arm was therefore closed. Results here are based upon a median follow-up of 18.9 months.
Results
Between 6/2014 and 2/2017, 245 patients, median age 76 years (range 60-88) entered the randomisation. Overall 60% were male; 66% had de Novo AML, 28% secondary AML, and 6% high risk MDS; 1% had favourable, 73% intermediate and 26% adverse cytogenetics. By validated Wheatley index, 2% were good risk, 36% standard risk and 63% poor risk. A median of 2 courses was delivered in either arm (mean 2.9 LDAC+tosedostat vs 2.3 LDAC).
Overall, complete remission was achieved in 18% of patients (LDAC+tosedostat 22%, LDAC 14%, OR 0.59 (0.31-1.13) p=0.11). Thirty-day mortality was not significantly increased (17% vs 13%, HR 1.44 (0.75-2.78) p=0.3); but overall survival showed no difference (2-year OS 16% vs 12%, HR 0.99 (0.74-1.32) p=0.9). Causes of death were: resistant/recurrent disease 39 vs 61; infection 20 vs 10; haemorrhage 8 vs 0; cardiac 5 vs 3; multiple 13 vs 8; other/unknown 5 vs 11. Relapse-free survival did not significantly differ (HR 0.93 (0.41-2.16) p=0.9) with median OS 28.4m vs 24.0m in responders (p=0.6); non-remitters had median OS 2.8m vs 3.3m (HR 1.20 (0.88-1.63) p=0.2). Stratified analyses failed to identify any subgroup of patients benefitting from tosedostat. Although rates of grade 3+ toxicity were low, tosedostat was associated with diarrhoea, increased cardiac toxicity, and increade use of platelets (mean 5.0 vs 3.5 p=0.006).

Conclusion
Despite promising early data and acceptable tolerability, we did not find evidence that the addition of tosedostat to LDAC produced a survival benefit in this group of patients, with the anticipated hazard ratio of 0.70 being outside the 95% confidence intervals at second interim analysis. Acknowledgements: We are grateful to CTI Biopharma for providing drug and support for this Investigator Initiated Study.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): AML, Clinical Trial


