Abstract: PB2183
Type: Publication Only
Background
The optimal intensity of myeloablation with a reduced-toxicity conditioning (RTC) regimen to decrease relapse rate after allogeneic stem cell transplant (allo-SCT)without increasing non-relapse mortality (NRM), has not been well established.
Aims
In this retrospective study at the American University of Beirut medical center (AUBMC) we aimed to evaluate the outcomes of patients who underwent allo-SCT with thiotepa, busulfan and fludarabine (TBF) as RTC.
Methods
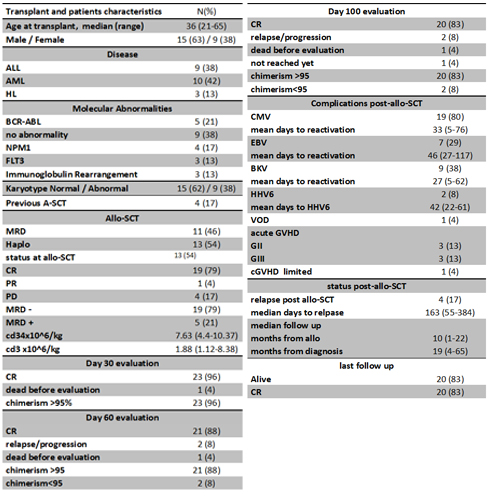
:We included twenty fourconsecutivepatients with hematological malignancies who received TBF as conditioning forallo-SCTfrom January to December 2016. All patients and transplant characteristics are listed in Table1.All patients received the myeloablative conditioning regimen consisting ofthiotepa(5mg/kg/day)infused on day -7 and -6, fludarabine(30mg/m2/day) was infused on day -5 to day -2; and busulfan(130mg/m2/day) was infused on day -5 to day-3. All patients received 2.5mg/kg/day intravenousrabbit antithymocyte globulin (ATG) on days -2 and -1. GVHD prophylaxis for patients transplanted from haploidentical donors consisted of post-transplant cyclophosphamide 50mg/kg/day on day +3 and day +5, cyclosporine started at 3 mg/kg/day on day+6 and readjusted according to level, and mycophenolate mofetil 500mgx4/day started on day+6 to +28. Patients transplanted from matched related donor, received cyclosporine as of day +1.
Results
Twenty three patients(96%) engrafted, with 14 days (range, 10-18) and 13 days (range, 8-48) as median time for neutrophil and platelet engraftment respectively. One patient who underwent haploidentical donor transplant with persistent disease for AML (karyotype 45,XY,-7) failed to engraft and died due to disease progression on day+22.After a median follow up of 10 months (range, 1-22) post-allo-SCT, the cumulative incidence of GradeII-IV acute GVHD (aGVHD) was 26%. One patient developed chronic limited GVHD (cGVHD).All the complication post allo-SCT are listed in table 1. Five patients (24%) relapsed post allo-SCT at a median of 163 days (range, 55-384), of which three (13%) died due to disease progression, and two were successfully salvaged and are in complete remission (CR) with full donor chimerism (FDC) at last follow up. Two patients developed JC virus progressive multifocal leukoencephalopathy, one of them made a full recovery and the other died in CR. The day 100 NRM was 0%. At last follow up 20 patients (83%) are alive in CR, with negative minimal residual disease and FDC.
Conclusion
Our results show that This TBF conditioning regimen appears to be safe,allows high rate of engraftment and low NRM rate among high-risk patients and can lead to a long-term disease control.
Session topic: 22. Stem cell transplantation - Clinical
Keyword(s): lymphoma, Leukemia, Haploidentical, Allogeneic hematopoietic stem cell transplant





