
Contributions
Abstract: PB2135
Type: Publication Only
Background
Lenalidomide maintenance therapy after autologous hematopoietic stem cell transplant (auto-HSCT) in the first-line treatment has been shown to improve progression-free survival (PFS) and overall survival (OS) in multiple myeloma (MM) patients.
Aims
This study assessed the budget impact of the United States (U.S.) Food and Drug Administration (FDA) approval of lenalidomide maintenance therapy on total healthcare costs of a U.S. health plan.
Methods
An economic model was developed to estimate the incremental (additional) total plan costs (in 2016 USD) of maintenance therapy in each year for the first 3 years after lenalidomide monotherapy (R) maintenance therapy approval. The number of post auto-HSCT adult MM pts eligible for initiating maintenance therapy was estimated from published epidemiological data and an analysis of Connect® MM Registry data. Clinical endpoints for R-maintenance, including time on treatment, PFS and OS, were obtained from a meta-analysis of published clinical trials (CALGB, IFM, and GIMEMA). The use of common off-label maintenance therapies was considered. Types of costs included in the model were drug, drug administration, adverse events (AE), AE monitoring, one-time progression and terminal care costs.
Results
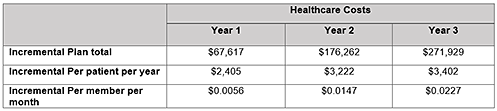
In a hypothetical U.S. health plan with 1 million members, the number of adult MM pts eligible to initiate post-ASCT maintenance therapy was estimated to be 28. Among them, 14.8 pts initiated R-maintenance in Year 1, 15.2 in Year 2, and 15.3 in Year 3, representing an incremental increase of 2.9%, 4.2% and 4.4% after R-maintenance therapy approval, respectively. After considering additional costs of maintenance, as well as potential offsets resulting from delayed progression the incremental total healthcare costs by year are listed in the table. Results were consistent across all total plan, per patient per year, and per member per month costs. Deterministic sensitivity analysis showed that the model results were robust to the variations of key model inputs.
Conclusion
Approval of lenalidomide monotherapy for maintenance after auto-HSCT in the first-line treatment of MM has minimal impact on total plan costs, primarily due to the small incident population and the already common use of lenalidomide in post auto-HSCT maintenance.
Session topic: 35. Quality of life, palliative care, ethics and health economics
Keyword(s): Multiple Myeloma, Maintenance, Immunomodulatory thalidomide analog, Health care
Abstract: PB2135
Type: Publication Only
Background
Lenalidomide maintenance therapy after autologous hematopoietic stem cell transplant (auto-HSCT) in the first-line treatment has been shown to improve progression-free survival (PFS) and overall survival (OS) in multiple myeloma (MM) patients.
Aims
This study assessed the budget impact of the United States (U.S.) Food and Drug Administration (FDA) approval of lenalidomide maintenance therapy on total healthcare costs of a U.S. health plan.
Methods
An economic model was developed to estimate the incremental (additional) total plan costs (in 2016 USD) of maintenance therapy in each year for the first 3 years after lenalidomide monotherapy (R) maintenance therapy approval. The number of post auto-HSCT adult MM pts eligible for initiating maintenance therapy was estimated from published epidemiological data and an analysis of Connect® MM Registry data. Clinical endpoints for R-maintenance, including time on treatment, PFS and OS, were obtained from a meta-analysis of published clinical trials (CALGB, IFM, and GIMEMA). The use of common off-label maintenance therapies was considered. Types of costs included in the model were drug, drug administration, adverse events (AE), AE monitoring, one-time progression and terminal care costs.
Results
In a hypothetical U.S. health plan with 1 million members, the number of adult MM pts eligible to initiate post-ASCT maintenance therapy was estimated to be 28. Among them, 14.8 pts initiated R-maintenance in Year 1, 15.2 in Year 2, and 15.3 in Year 3, representing an incremental increase of 2.9%, 4.2% and 4.4% after R-maintenance therapy approval, respectively. After considering additional costs of maintenance, as well as potential offsets resulting from delayed progression the incremental total healthcare costs by year are listed in the table. Results were consistent across all total plan, per patient per year, and per member per month costs. Deterministic sensitivity analysis showed that the model results were robust to the variations of key model inputs.

Conclusion
Approval of lenalidomide monotherapy for maintenance after auto-HSCT in the first-line treatment of MM has minimal impact on total plan costs, primarily due to the small incident population and the already common use of lenalidomide in post auto-HSCT maintenance.
Session topic: 35. Quality of life, palliative care, ethics and health economics
Keyword(s): Multiple Myeloma, Maintenance, Immunomodulatory thalidomide analog, Health care


