
Contributions
Abstract: S818
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 09:00 - 09:15
Location: Room N111
Background
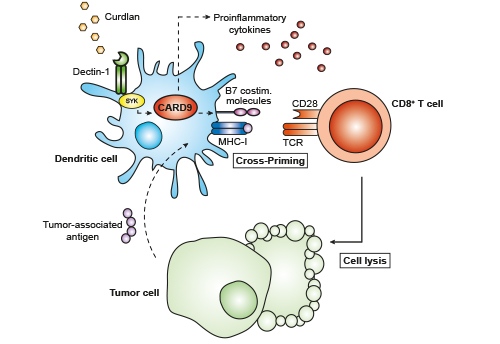
Activation of the C-type lectin receptor Dectin-1 by beta-glucans triggers multiple signals within dendritic cells (DCs) that result in activation of innate immunity. While these mechanisms can potently prime CD8+ cytotoxic T cell (CTL) responses without additional adjuvants, the Dectin‑1 effector pathways that control CTL induction remain unclear.
Aims
Aims of this study were:
Methods
We used in vitro coculture between DCs (wildtype vs gene deficient) and CD8 T cells to define signalling components of Dectin-1 induced CTL cross-priming.
Results
Here we demonstrate that Dectin‑1-induced CTL cross-priming in mice does not require inflammasome activation but strictly depends on the adapter protein Card9 in vitro. In vivo, Dectin-1-mediated Card9 activation after vaccination drives both expansion and activation of antigen-specific CTLs, resulting in long-lasting CTL responses which are sufficient to protect mice from tumor challenge. This Dectin-1-induced antitumor immune response was independent of natural killer (NK) cell function and completely abrogated in Card9-deficient mice. Thus, our results demonstrate that Dectin-1-triggered Card9 signaling but not inflammasome activation can potently cross-prime antigen specific CTLs, suggesting that this pathway would be a candidate for immunotherapy and vaccine development.
Conclusion
We identify Card9 as central regulator of Dectin-1-induced cross-priming of cytotoxic T cells (CTLs) in mice. These antigen specific CTLs mediate potent antitumor immunity independent of inflammasome activity and NK cells. This pathway is a candidate for immunotherapy and vaccine development.
Session topic: 24. Gene therapy, cellular immunotherapy and vaccination
Keyword(s): Tumor immunity, T cell activation, Signaling molecules, Cytotoxic T cell
Abstract: S818
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 09:00 - 09:15
Location: Room N111
Background
Activation of the C-type lectin receptor Dectin-1 by beta-glucans triggers multiple signals within dendritic cells (DCs) that result in activation of innate immunity. While these mechanisms can potently prime CD8+ cytotoxic T cell (CTL) responses without additional adjuvants, the Dectin‑1 effector pathways that control CTL induction remain unclear.
Aims
Aims of this study were:
Methods
We used in vitro coculture between DCs (wildtype vs gene deficient) and CD8 T cells to define signalling components of Dectin-1 induced CTL cross-priming.
Results
Here we demonstrate that Dectin‑1-induced CTL cross-priming in mice does not require inflammasome activation but strictly depends on the adapter protein Card9 in vitro. In vivo, Dectin-1-mediated Card9 activation after vaccination drives both expansion and activation of antigen-specific CTLs, resulting in long-lasting CTL responses which are sufficient to protect mice from tumor challenge. This Dectin-1-induced antitumor immune response was independent of natural killer (NK) cell function and completely abrogated in Card9-deficient mice. Thus, our results demonstrate that Dectin-1-triggered Card9 signaling but not inflammasome activation can potently cross-prime antigen specific CTLs, suggesting that this pathway would be a candidate for immunotherapy and vaccine development.

Conclusion
We identify Card9 as central regulator of Dectin-1-induced cross-priming of cytotoxic T cells (CTLs) in mice. These antigen specific CTLs mediate potent antitumor immunity independent of inflammasome activity and NK cells. This pathway is a candidate for immunotherapy and vaccine development.
Session topic: 24. Gene therapy, cellular immunotherapy and vaccination
Keyword(s): Tumor immunity, T cell activation, Signaling molecules, Cytotoxic T cell


