INTRAVENOUS IRON VERSUS ORAL IRON VERSUS NO IRON WITH OR WITHOUT ERYTHROPOIESISSTIMULATING AGENTS (ESA) FOR CANCER PATIENTS WITH ANAEMIA: A SYSTEMATIC REVIEW AND NETWORK META-ANALYSIS
(Abstract release date: 05/18/17)
EHA Library. Weigl A. 06/25/17; 182099; S812

Aaron Weigl
Contributions
Contributions
Abstract
Abstract: S812
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:45 - 09:00
Location: Room N109
Background
A widely prevalent complication in patients suffering from cancer is the deficiency of haemoglobin-containing red blood cells, referred to as anaemia. While many patients develop anaemia due to an involvement of malignant bone marrow cells, others suffer from so called chemotherapy/radiotherapy-induced anemia. Erythropoiesis-stimulating agents (ESAs) stimulate the production of red blood cells within the bone marrow and have shown to increase Hb levels in anaemic patients. Uncertainties remain regarding the effect of iron supplementation on the fatal consequences of ESA-treatment.
Aims
The aims of this systematic review and network meta-analysis are to evaluate benefits and risks of ESAs and iron for the treatment of disease-related as well as therapy induced anaemia in cancer patients.
Methods
Based on an a-priori Cochrane protocol, we developed sensitive search strategies for Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, databases of ongoing trials and conference proceedings (search date 12/2016). We included only randomized controlled trials (RCTs) including anaemic patients of any age with solid and/or haematological malignancy undergoing chemotherapy, radiotherapy or no anti-cancer therapy. We excluded studies including anaemic cancer-patients as a result of surgery or due to haemolysis. Two authors independently assessed studies for eligibility, extracted data and assessed quality of trials. The primary outcome was on-study mortality. Secondary outcomes included number of red blood cell transfusions and thromboembolic events. For binary outcomes, we used risk ratios (RRs) with corresponding 95% confidence intervals (CIs) to evaluate the treatment effects. We performed a random-effects meta-analysis for direct comparisons. For network meta-analyses, we used the frequentist graph-theoretical approach. Treatment hierarchy was obtained giving P-scores on a scale from 0 (worst) to 1 (best).
This project was funded by the Federal Ministry of Education and Research, grant number: 01KG1405
Results
We identified a total number of 105 eligible studies, including 25.722 patients. The network analysis of the primary outcome, on-study mortality, included 69 studies and 8 treatments. As the given network was not fully connected, we performed pairwise comparisons on the four subnetworks with 2 treatments each. Statistically significant treatment disadvantages were shown in the direct comparison of ESA plus iron supplementation (given if necessary) compared to placebo/no treatment plus iron supplementation (given if necessary) (RR 1.14 (95% CI 1.03-1.25), including 41 studies).
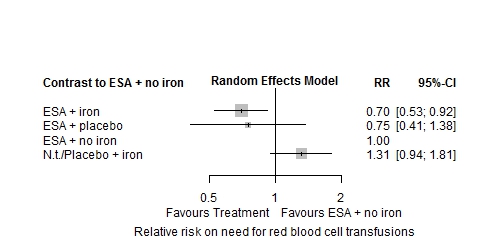
Network meta-analysis on the need for red blood cell (RBC) transfusions showed the treatment of ESA plus iron supplementation to have the most positive effect compared to ESA alone (RR: 0.70 (95% CI 0.53-0.92) P-score: 0.87). No relevant heterogeneity was found within the analysed network of four treatments (I²=18.4%). Inconsistency could not be tested statistically as no closed loop was included.
Thromboembolic events occurred most often in patients treated with ESAs, irrespective of iron supplementation (ESA plus iron vs. no treatment/placebo plus no iron: RR 1.79 (95% CI 0.74-4.32) P-score: 0.22, ESA plus no iron vs. no treatment/placebo plus no iron: RR 1.90 (95% CI 0.96-3.75) P-score: 0.16). Subgroup analysis regarding type of iron, as well as route of administration will be presented at the EHA-congress.
Conclusion
While our analyses show that ESA use increases mortality and risk for thromboembolic events, there is no evidence that iron supplementation alters these risks. However, addition of iron to ESA does further decrease the need for RBC-transfusions compared to ESA alone. Further investigation, with regards to iron type and route of administration may yield further distinct results.
Session topic: 28. Iron metabolism, deficiency and overload
Keyword(s): Iron, Epoetin, Cancer, Anemia
Abstract: S812
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:45 - 09:00
Location: Room N109
Background
A widely prevalent complication in patients suffering from cancer is the deficiency of haemoglobin-containing red blood cells, referred to as anaemia. While many patients develop anaemia due to an involvement of malignant bone marrow cells, others suffer from so called chemotherapy/radiotherapy-induced anemia. Erythropoiesis-stimulating agents (ESAs) stimulate the production of red blood cells within the bone marrow and have shown to increase Hb levels in anaemic patients. Uncertainties remain regarding the effect of iron supplementation on the fatal consequences of ESA-treatment.
Aims
The aims of this systematic review and network meta-analysis are to evaluate benefits and risks of ESAs and iron for the treatment of disease-related as well as therapy induced anaemia in cancer patients.
Methods
Based on an a-priori Cochrane protocol, we developed sensitive search strategies for Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, databases of ongoing trials and conference proceedings (search date 12/2016). We included only randomized controlled trials (RCTs) including anaemic patients of any age with solid and/or haematological malignancy undergoing chemotherapy, radiotherapy or no anti-cancer therapy. We excluded studies including anaemic cancer-patients as a result of surgery or due to haemolysis. Two authors independently assessed studies for eligibility, extracted data and assessed quality of trials. The primary outcome was on-study mortality. Secondary outcomes included number of red blood cell transfusions and thromboembolic events. For binary outcomes, we used risk ratios (RRs) with corresponding 95% confidence intervals (CIs) to evaluate the treatment effects. We performed a random-effects meta-analysis for direct comparisons. For network meta-analyses, we used the frequentist graph-theoretical approach. Treatment hierarchy was obtained giving P-scores on a scale from 0 (worst) to 1 (best).
This project was funded by the Federal Ministry of Education and Research, grant number: 01KG1405
Results
We identified a total number of 105 eligible studies, including 25.722 patients. The network analysis of the primary outcome, on-study mortality, included 69 studies and 8 treatments. As the given network was not fully connected, we performed pairwise comparisons on the four subnetworks with 2 treatments each. Statistically significant treatment disadvantages were shown in the direct comparison of ESA plus iron supplementation (given if necessary) compared to placebo/no treatment plus iron supplementation (given if necessary) (RR 1.14 (95% CI 1.03-1.25), including 41 studies).
Network meta-analysis on the need for red blood cell (RBC) transfusions showed the treatment of ESA plus iron supplementation to have the most positive effect compared to ESA alone (RR: 0.70 (95% CI 0.53-0.92) P-score: 0.87). No relevant heterogeneity was found within the analysed network of four treatments (I²=18.4%). Inconsistency could not be tested statistically as no closed loop was included.
Thromboembolic events occurred most often in patients treated with ESAs, irrespective of iron supplementation (ESA plus iron vs. no treatment/placebo plus no iron: RR 1.79 (95% CI 0.74-4.32) P-score: 0.22, ESA plus no iron vs. no treatment/placebo plus no iron: RR 1.90 (95% CI 0.96-3.75) P-score: 0.16). Subgroup analysis regarding type of iron, as well as route of administration will be presented at the EHA-congress.

Conclusion
While our analyses show that ESA use increases mortality and risk for thromboembolic events, there is no evidence that iron supplementation alters these risks. However, addition of iron to ESA does further decrease the need for RBC-transfusions compared to ESA alone. Further investigation, with regards to iron type and route of administration may yield further distinct results.
Session topic: 28. Iron metabolism, deficiency and overload
Keyword(s): Iron, Epoetin, Cancer, Anemia
{{ help_message }}
{{filter}}


