
Contributions
Abstract: S784
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:00 - 08:15
Location: Hall E
Background
Polycythemia vera (PV) is characterized by hyperproliferation of erythroid/myeloid/megakaryocytic components in the bone marrow, cardiovascular complications, and high symptom burden. Treatment (Tx) in patients (pts) with PV is focused on maintaining hematocrit (HCT) level <45%. RESPONSE-2 study evaluated the efficacy and safety of ruxolitinib (RUX) vs best available therapy (BAT) in hydroxyurea (HU)-resistant/intolerant pts with PV ≥18 years without splenomegaly and with phlebotomy [PBT] requirement to control HCT. At week (wk) 28 (primary analysis), HCT control was reported in 46/74 pts in the RUX arm vs 14/75 pts in the BAT arm.
Aims
This preplanned analysis of RESPONSE-2 evaluated the durability of efficacy and safety of RUX vs BAT, after all pts reached 80 wk into the study or discontinued the study.
Methods
Pts were randomized 1:1 to RUX 10 mg twice daily or BAT. Primary end point was the proportion of pts who achieved HCT control at wk 28 (absence of PBT eligibility [HCT >45%, ie, ≥3 percentage points from baseline, or HCT >48%] from wk 8 to 28, with ≤1 PBT eligibility from wk 0 to 8). Key secondary end point was the proportion of pts who achieved complete hematologic remission at wk 28 (CHR: HCT <45%, WBC ≤10 x 109/L, platelet count ≤400 x 109/L). Durability of HCT, CHR, and safety was evaluated at wk 80 (data cutoff, September 26, 2016). Additional end points included assessment of patient-reported outcomes (MPN-SAF TSS) and change in JAK2V617F allele burden over time. BAT pts could cross over to RUX from wk 28.
Results
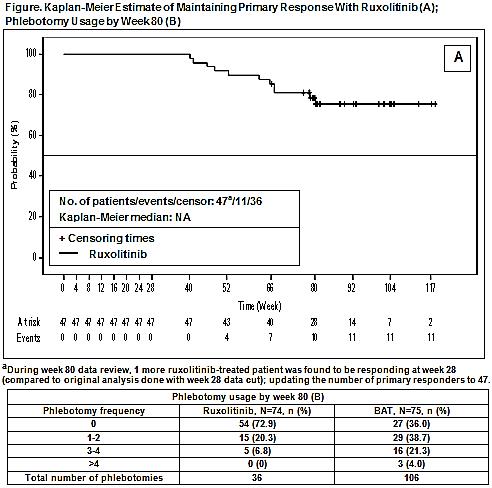
Baseline demographics were comparable among RUX (N=74) and BAT (N=75) arms. When last pt reached wk 80 time point, 69 pts randomized to RUX were still receiving Tx; while 5 pts discontinued Tx (adverse events [AEs]=3 pts, physician’s decision/pt withdrew consent=1 pt, each). In BAT arm, 58 pts crossed over to RUX (crossover data to be included in presentation) with remaining pts either ongoing follow-up (f/u [n=5]) or having discontinued Tx (completed f/u per protocol, n=7; death, n=1; other reasons, n=4). Median exposure was 93.6 wk in RUX vs 28.4 wk in the BAT arm. At wk 80, durable HCT control was achieved in 35 pts (47%) in RUX vs 2 pts (3%) in BAT arm. Of those who achieved a HCT response at wk 28, Kaplan-Meier estimate of maintaining response up to wk 80 was 78.37% in the RUX arm. Durable CHR was achieved in 18 pts (24%) in RUX vs 2 pts (3%) in the BAT arm. Total number of PBT was higher in the BAT arm vs RUX arm (Table). At wk 80, 45% of pts randomized to RUX continued to achieve a ≥50% of reduction in the MPN-SAF TSS. At wk 80, mean percentage change from baseline in JAK2V617F allele burden was −9.7% in the RUX (n=65) vs +0.3% in the BAT arm (n=3). AEs observed were consistent with those generally reported with RUX (primarily low grade [G]). Most common AEs (all G, exposure-adjusted rate per 100 pt-years) were anemia (14.3), weight increase (10.6), arthralgia (9.1), and pruritus (9.1) in the RUX arm vs pruritus (37.5), headache (16.9), and thrombocytopenia (15.0) in the BAT arm. Rate of thromboembolic events (Standardized MedDRA Query, exposure-adjusted) was RUX (1.5) vs BAT arm (1.9). No pt in the RUX arm had disease progression vs 2 pts in the BAT arm. No deaths were reported in the RUX arm vs 3 pts in the BAT arm (septic shock/disease progression/study indication=1 pt, each).
Conclusion
RUX provided durable HCT control, durable CHR, reduction in PBT requirement, improved symptom burden, and was generally well tolerated with >90% of pts still receiving Tx at wk 80. RUX Tx provided a modest reduction in allele burden over time. Findings from both RESPONSE studies suggest that RUX should be considered as a standard of care for second-line Tx in this inadequately controlled pt population with PV.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Ruxolitinib, Polycythemia vera, Myeloproliferative disorder
Abstract: S784
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:00 - 08:15
Location: Hall E
Background
Polycythemia vera (PV) is characterized by hyperproliferation of erythroid/myeloid/megakaryocytic components in the bone marrow, cardiovascular complications, and high symptom burden. Treatment (Tx) in patients (pts) with PV is focused on maintaining hematocrit (HCT) level <45%. RESPONSE-2 study evaluated the efficacy and safety of ruxolitinib (RUX) vs best available therapy (BAT) in hydroxyurea (HU)-resistant/intolerant pts with PV ≥18 years without splenomegaly and with phlebotomy [PBT] requirement to control HCT. At week (wk) 28 (primary analysis), HCT control was reported in 46/74 pts in the RUX arm vs 14/75 pts in the BAT arm.
Aims
This preplanned analysis of RESPONSE-2 evaluated the durability of efficacy and safety of RUX vs BAT, after all pts reached 80 wk into the study or discontinued the study.
Methods
Pts were randomized 1:1 to RUX 10 mg twice daily or BAT. Primary end point was the proportion of pts who achieved HCT control at wk 28 (absence of PBT eligibility [HCT >45%, ie, ≥3 percentage points from baseline, or HCT >48%] from wk 8 to 28, with ≤1 PBT eligibility from wk 0 to 8). Key secondary end point was the proportion of pts who achieved complete hematologic remission at wk 28 (CHR: HCT <45%, WBC ≤10 x 109/L, platelet count ≤400 x 109/L). Durability of HCT, CHR, and safety was evaluated at wk 80 (data cutoff, September 26, 2016). Additional end points included assessment of patient-reported outcomes (MPN-SAF TSS) and change in JAK2V617F allele burden over time. BAT pts could cross over to RUX from wk 28.
Results
Baseline demographics were comparable among RUX (N=74) and BAT (N=75) arms. When last pt reached wk 80 time point, 69 pts randomized to RUX were still receiving Tx; while 5 pts discontinued Tx (adverse events [AEs]=3 pts, physician’s decision/pt withdrew consent=1 pt, each). In BAT arm, 58 pts crossed over to RUX (crossover data to be included in presentation) with remaining pts either ongoing follow-up (f/u [n=5]) or having discontinued Tx (completed f/u per protocol, n=7; death, n=1; other reasons, n=4). Median exposure was 93.6 wk in RUX vs 28.4 wk in the BAT arm. At wk 80, durable HCT control was achieved in 35 pts (47%) in RUX vs 2 pts (3%) in BAT arm. Of those who achieved a HCT response at wk 28, Kaplan-Meier estimate of maintaining response up to wk 80 was 78.37% in the RUX arm. Durable CHR was achieved in 18 pts (24%) in RUX vs 2 pts (3%) in the BAT arm. Total number of PBT was higher in the BAT arm vs RUX arm (Table). At wk 80, 45% of pts randomized to RUX continued to achieve a ≥50% of reduction in the MPN-SAF TSS. At wk 80, mean percentage change from baseline in JAK2V617F allele burden was −9.7% in the RUX (n=65) vs +0.3% in the BAT arm (n=3). AEs observed were consistent with those generally reported with RUX (primarily low grade [G]). Most common AEs (all G, exposure-adjusted rate per 100 pt-years) were anemia (14.3), weight increase (10.6), arthralgia (9.1), and pruritus (9.1) in the RUX arm vs pruritus (37.5), headache (16.9), and thrombocytopenia (15.0) in the BAT arm. Rate of thromboembolic events (Standardized MedDRA Query, exposure-adjusted) was RUX (1.5) vs BAT arm (1.9). No pt in the RUX arm had disease progression vs 2 pts in the BAT arm. No deaths were reported in the RUX arm vs 3 pts in the BAT arm (septic shock/disease progression/study indication=1 pt, each).

Conclusion
RUX provided durable HCT control, durable CHR, reduction in PBT requirement, improved symptom burden, and was generally well tolerated with >90% of pts still receiving Tx at wk 80. RUX Tx provided a modest reduction in allele burden over time. Findings from both RESPONSE studies suggest that RUX should be considered as a standard of care for second-line Tx in this inadequately controlled pt population with PV.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Ruxolitinib, Polycythemia vera, Myeloproliferative disorder


