
Contributions
Abstract: S776
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:30 - 08:45
Location: Hall C
Background
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma (iNHL) subtype, yet treatment options in the relapsed/refractory setting are limited. Copanlisib is a potent and selective pan-class I PI3K inhibitor with predominant activity against the δ- and α-isoforms.
Aims
We report results from the FL subset of a large phase II study in iNHL patients (NCT01660451, part B).
Methods
Patients with histologically confirmed indolent indolent FL (grade 1-3a) relapsed/refractory to ≥2 prior lines of treatment were treated with copanlisib (60 mg IV infusion) administered on days 1, 8 and 15 of a 28-day cycle until disease progression or unacceptable toxicity. The primary endpoint was objective response rate (ORR) as assessed by independent radiology review according to the response criteria for lymphoma (Cheson et al, JCO 20:579, 2007). Secondary endpoints included progression-free survival (PFS) and duration of response (DOR), safety and tolerability.
Results
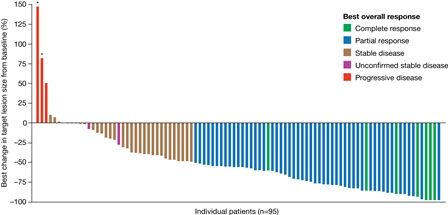
A total of 141 patients with iNHL were treated in the phase II study, including 104 patients with FL. The FL subset was characterized as: 52% male, 83% white, median age 62 yrs, 62% ECOG 0, 63% refractory to last therapy, median time from most recent progression 8 wks (range 1-73) and median prior lines of therapy 3 (range 2-8). At the time of primary analysis the ORR was 58.7%, comprising 15 patients (14.4%) with complete response and 46 (44.2%) with partial response. Stable disease was observed in 35 (33.7%) patients and progression of disease as best response in 2 patients. The median duration of response was 370 days (range 0-687), with 43 responders censored at data cut-off. Median duration of treatment was 22 wks (range 1-105); 33 (32%) patients remained on treatment. Per investigator assessment, 87 of 96 evaluable patients (91%) had some degree of tumor shrinkage as best response, and 58/96 (60%) had >50% tumor shrinkage (Figure). For all patients in the phase II study, the most common treatment-emergent AEs occurring in >25% of patients included (all grade/grade 3+): diarrhea (34%/5%), reduced neutrophil count (30%/24%), fatigue (30%/2%), and fever (25%/4%). Hyperglycemia (50%/41%) and hypertension (30%/24%) were transient. The incidence of pneumonitis (8%/1.4%), hepatic enzymopathy (AST 28%/1.4%; ALT 23%/1.4%), opportunistic infection (1.4%) and colitis (0.7%) were low. Six deaths were observed, 3 of which were attributed to copanlisib: one lung infection, one respiratory failure, and one thromboembolic event.
Conclusion
Copanlisib was highly active as a single agent in heavily pretreated relapsed/refractory FL patients and resulted in responses in the majority of patients, with a median duration of response exceeding one year. Toxicities were manageable, with a low incidence of severe AEs associated with other PI3K inhibitors, especially hepatic enzymopathy, opportunistic infections, and colitis.
Session topic: 19. Indolent Non-Hodgkin lymphoma - Clinical
Keyword(s): PI3K, Follicular lymphoma
Abstract: S776
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:30 - 08:45
Location: Hall C
Background
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma (iNHL) subtype, yet treatment options in the relapsed/refractory setting are limited. Copanlisib is a potent and selective pan-class I PI3K inhibitor with predominant activity against the δ- and α-isoforms.
Aims
We report results from the FL subset of a large phase II study in iNHL patients (NCT01660451, part B).
Methods
Patients with histologically confirmed indolent indolent FL (grade 1-3a) relapsed/refractory to ≥2 prior lines of treatment were treated with copanlisib (60 mg IV infusion) administered on days 1, 8 and 15 of a 28-day cycle until disease progression or unacceptable toxicity. The primary endpoint was objective response rate (ORR) as assessed by independent radiology review according to the response criteria for lymphoma (Cheson et al, JCO 20:579, 2007). Secondary endpoints included progression-free survival (PFS) and duration of response (DOR), safety and tolerability.
Results
A total of 141 patients with iNHL were treated in the phase II study, including 104 patients with FL. The FL subset was characterized as: 52% male, 83% white, median age 62 yrs, 62% ECOG 0, 63% refractory to last therapy, median time from most recent progression 8 wks (range 1-73) and median prior lines of therapy 3 (range 2-8). At the time of primary analysis the ORR was 58.7%, comprising 15 patients (14.4%) with complete response and 46 (44.2%) with partial response. Stable disease was observed in 35 (33.7%) patients and progression of disease as best response in 2 patients. The median duration of response was 370 days (range 0-687), with 43 responders censored at data cut-off. Median duration of treatment was 22 wks (range 1-105); 33 (32%) patients remained on treatment. Per investigator assessment, 87 of 96 evaluable patients (91%) had some degree of tumor shrinkage as best response, and 58/96 (60%) had >50% tumor shrinkage (Figure). For all patients in the phase II study, the most common treatment-emergent AEs occurring in >25% of patients included (all grade/grade 3+): diarrhea (34%/5%), reduced neutrophil count (30%/24%), fatigue (30%/2%), and fever (25%/4%). Hyperglycemia (50%/41%) and hypertension (30%/24%) were transient. The incidence of pneumonitis (8%/1.4%), hepatic enzymopathy (AST 28%/1.4%; ALT 23%/1.4%), opportunistic infection (1.4%) and colitis (0.7%) were low. Six deaths were observed, 3 of which were attributed to copanlisib: one lung infection, one respiratory failure, and one thromboembolic event.

Conclusion
Copanlisib was highly active as a single agent in heavily pretreated relapsed/refractory FL patients and resulted in responses in the majority of patients, with a median duration of response exceeding one year. Toxicities were manageable, with a low incidence of severe AEs associated with other PI3K inhibitors, especially hepatic enzymopathy, opportunistic infections, and colitis.
Session topic: 19. Indolent Non-Hodgkin lymphoma - Clinical
Keyword(s): PI3K, Follicular lymphoma


