
Contributions
Abstract: S773
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 09:00 - 09:15
Location: Hall A
Background
Aims
The primary aim of the study was to understand the safety and activity of cerdulatinib in B-cell malignancies.
Methods
This is an open-label study with 28-day cycles. Twice daily (BID; 30 mg and 35 mg) dosing was evaluated. Pharmacokinetics (PK), pharmacodynamics (PD), and safety were monitored, as well as an assessment of efficacy. Clinical response was assessed by standard criteria. Potency and specificity for SYK and JAK pathway inhibition were measured in whole blood assays by monitoring signaling responses following ligation of the BCR and receptors for IL-4. Serum markers of inflammation, minimal residual disease (MRD) and apoptosis in CLL patients were also measured.
Results
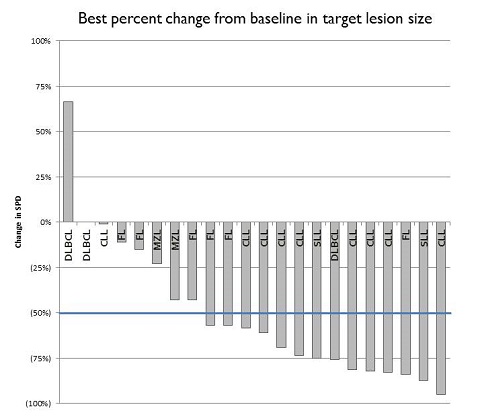
A phase 2 study was initiated in May 2016 to enroll up to 40 patients in each of three cohorts; 1) relapsed/refractory CLL/SLL, 2) relapsed/refractory indolent NHL, and 3) relapsed DLBCL, MCL and transformed FL. As of March 1, 2017, 37 patients have been enrolled, 17 with CLL/SLL, 15 with indolent NHL (10 FL, 4 MZL, 1 WM), and 5 with aggressive NHL (3 DLBCL, 1 MCL, 1 tFL). Median patient age is 70 years (range, 51-93). The median number of prior therapies is 3 (range 1–7). 11 patients had prior BTK or PI3K inhibitor therapy.
Conclusion
Cerdulatinib demonstrates clinical activity in heavily pretreated patients with CLL/B-cell NHL and is generally well tolerated. Consistent activity is seen in patients with CLL and FL. Accrual is proceeding; updated PK/PD, safety and efficacy will be presented.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Abstract: S773
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 09:00 - 09:15
Location: Hall A
Background
Aims
The primary aim of the study was to understand the safety and activity of cerdulatinib in B-cell malignancies.
Methods
This is an open-label study with 28-day cycles. Twice daily (BID; 30 mg and 35 mg) dosing was evaluated. Pharmacokinetics (PK), pharmacodynamics (PD), and safety were monitored, as well as an assessment of efficacy. Clinical response was assessed by standard criteria. Potency and specificity for SYK and JAK pathway inhibition were measured in whole blood assays by monitoring signaling responses following ligation of the BCR and receptors for IL-4. Serum markers of inflammation, minimal residual disease (MRD) and apoptosis in CLL patients were also measured.
Results
A phase 2 study was initiated in May 2016 to enroll up to 40 patients in each of three cohorts; 1) relapsed/refractory CLL/SLL, 2) relapsed/refractory indolent NHL, and 3) relapsed DLBCL, MCL and transformed FL. As of March 1, 2017, 37 patients have been enrolled, 17 with CLL/SLL, 15 with indolent NHL (10 FL, 4 MZL, 1 WM), and 5 with aggressive NHL (3 DLBCL, 1 MCL, 1 tFL). Median patient age is 70 years (range, 51-93). The median number of prior therapies is 3 (range 1–7). 11 patients had prior BTK or PI3K inhibitor therapy.

Conclusion
Cerdulatinib demonstrates clinical activity in heavily pretreated patients with CLL/B-cell NHL and is generally well tolerated. Consistent activity is seen in patients with CLL and FL. Accrual is proceeding; updated PK/PD, safety and efficacy will be presented.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical


