
Contributions
Abstract: S502
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:15 - 16:30
Location: Room N109
Background
Maintenance of pretreatment health-related quality of life (HRQoL) and/or meaningful improvements in HRQoL are important for previously untreated indolent non-Hodgkin lymphoma (iNHL) patients (pts). GALLIUM (NCT01332968) is an open-label, randomized Phase III study of obinutuzumab (GA101; G) plus chemotherapy (chemo) followed by G maintenance (G-chemo) compared with rituximab (R) plus chemo followed by R maintenance (R-chemo) in pts with previously untreated iNHL. In GALLIUM, G-chemo produced a clinically meaningful improvement in investigator-assessed progression-free survival (PFS) among follicular lymphoma (FL) pts (34% reduction in risk of a PFS event relative to R-chemo). Grade 3–5 and serious adverse events were more common with G-chemo.
Aims
To compare changes in HRQoL in FL pts receiving G-chemo and R-chemo during GALLIUM.
Methods
Enrolled pts were aged ≥18 years with documented, previously untreated FL (grades 1–3a), advanced disease (stage III/IV or stage II with tumor diameter ≥7cm), ECOG performance status 0–2, and requiring treatment according to GELF criteria. Pts were randomized 1:1 to R 375mg/m2 on day (D) 1 of each cycle (C) or G 1000mg on D1, 8, and 15 of C1 and D1 of C2–8, for 6 or 8 cycles depending on chemo (CHOP, CVP or bendamustine). Responders continued to receive R or G every 2 months (mo) for 2 years or until progression. The Functional Assessment of Cancer Treatment-Lymphoma (FACT-Lym) questionnaire (Webster et al. 2005) was used to assess overall HRQoL, physical and functional well-being, and disease- and treatment-related symptoms. FACT-Lym was administered on D1 of C1 and C3 during induction, at the end of induction, and at mo 2 and 12 during maintenance/follow-up. For each FACT-Lym scale, mean and 95% confidence interval (CI) were derived for recorded scores at each visit and changes from baseline. Minimally important differences (MIDs) were used to calculate the proportion of pts reporting improvement on the FACT-Lym lymphoma subscale (LYMS; ≥3 points), Trial Outcome Index (TOI; ≥6 points), and lymphoma total score (Lym-Total; ≥7 points). All pts gave informed consent.
Results
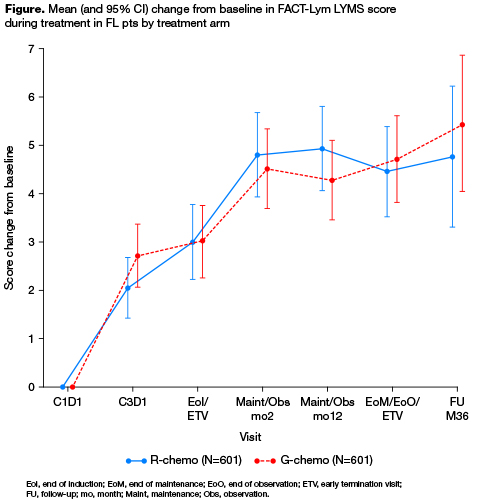
Of 1202 FL pts randomized (median age, 59 yrs; 53.2% female; median observation time, 34.5 mo [range 0–54.5]), 556/601 (92.5%; G-chemo) and 550/601 (91.5%; R-chemo) completed all FACT-Lym scales at baseline. Baseline demographics and disease characteristics were balanced between arms. At baseline, mean HRQoL scores were similar in the two treatment arms, with all pts having some impairment of physical function, functional wellbeing, emotional and social function. Over the course of treatment, mean HRQoL was similar in the two treatment arms. From end of induction onwards, pts in both arms experienced clinically meaningful improvements from baseline in LYMS scores (Figure), and the summary scales that included this subscale (TOI, Lym-Total). On each summary scale, ~50% of patients in each arm reported clinically meaningful improvements. There were no clear differences between arms in HRQoL scores over the course of therapy.
Conclusion
In previously untreated FL pts in GALLIUM, G-chemo and R-chemo produced similar improvements in HRQoL. These results suggest that lymphoma-related symptoms were reduced by both treatments and that the resulting improvements in well-being were not abrogated by treatment-related side effects. When viewed in the context of longer PFS, these results further support the relative benefit of G-chemo over R-chemo in GALLIUM.
Session topic: 35. Quality of life, palliative care, ethics and health economics
Keyword(s): Rituximab, Obinutuzumab, Indolent Non-Hodgkin's Lymphoma, Follicular lymphoma
Abstract: S502
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:15 - 16:30
Location: Room N109
Background
Maintenance of pretreatment health-related quality of life (HRQoL) and/or meaningful improvements in HRQoL are important for previously untreated indolent non-Hodgkin lymphoma (iNHL) patients (pts). GALLIUM (NCT01332968) is an open-label, randomized Phase III study of obinutuzumab (GA101; G) plus chemotherapy (chemo) followed by G maintenance (G-chemo) compared with rituximab (R) plus chemo followed by R maintenance (R-chemo) in pts with previously untreated iNHL. In GALLIUM, G-chemo produced a clinically meaningful improvement in investigator-assessed progression-free survival (PFS) among follicular lymphoma (FL) pts (34% reduction in risk of a PFS event relative to R-chemo). Grade 3–5 and serious adverse events were more common with G-chemo.
Aims
To compare changes in HRQoL in FL pts receiving G-chemo and R-chemo during GALLIUM.
Methods
Enrolled pts were aged ≥18 years with documented, previously untreated FL (grades 1–3a), advanced disease (stage III/IV or stage II with tumor diameter ≥7cm), ECOG performance status 0–2, and requiring treatment according to GELF criteria. Pts were randomized 1:1 to R 375mg/m2 on day (D) 1 of each cycle (C) or G 1000mg on D1, 8, and 15 of C1 and D1 of C2–8, for 6 or 8 cycles depending on chemo (CHOP, CVP or bendamustine). Responders continued to receive R or G every 2 months (mo) for 2 years or until progression. The Functional Assessment of Cancer Treatment-Lymphoma (FACT-Lym) questionnaire (Webster et al. 2005) was used to assess overall HRQoL, physical and functional well-being, and disease- and treatment-related symptoms. FACT-Lym was administered on D1 of C1 and C3 during induction, at the end of induction, and at mo 2 and 12 during maintenance/follow-up. For each FACT-Lym scale, mean and 95% confidence interval (CI) were derived for recorded scores at each visit and changes from baseline. Minimally important differences (MIDs) were used to calculate the proportion of pts reporting improvement on the FACT-Lym lymphoma subscale (LYMS; ≥3 points), Trial Outcome Index (TOI; ≥6 points), and lymphoma total score (Lym-Total; ≥7 points). All pts gave informed consent.
Results
Of 1202 FL pts randomized (median age, 59 yrs; 53.2% female; median observation time, 34.5 mo [range 0–54.5]), 556/601 (92.5%; G-chemo) and 550/601 (91.5%; R-chemo) completed all FACT-Lym scales at baseline. Baseline demographics and disease characteristics were balanced between arms. At baseline, mean HRQoL scores were similar in the two treatment arms, with all pts having some impairment of physical function, functional wellbeing, emotional and social function. Over the course of treatment, mean HRQoL was similar in the two treatment arms. From end of induction onwards, pts in both arms experienced clinically meaningful improvements from baseline in LYMS scores (Figure), and the summary scales that included this subscale (TOI, Lym-Total). On each summary scale, ~50% of patients in each arm reported clinically meaningful improvements. There were no clear differences between arms in HRQoL scores over the course of therapy.

Conclusion
In previously untreated FL pts in GALLIUM, G-chemo and R-chemo produced similar improvements in HRQoL. These results suggest that lymphoma-related symptoms were reduced by both treatments and that the resulting improvements in well-being were not abrogated by treatment-related side effects. When viewed in the context of longer PFS, these results further support the relative benefit of G-chemo over R-chemo in GALLIUM.
Session topic: 35. Quality of life, palliative care, ethics and health economics
Keyword(s): Rituximab, Obinutuzumab, Indolent Non-Hodgkin's Lymphoma, Follicular lymphoma


