
Contributions
Abstract: S495
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 17:00 - 17:15
Location: Room N103
Background
HLA-haploidentical allogeneic hematopoietic stem cell transplant (haplo-HSCT) offers an option for children with acute leukemia in need of a transplant and lacking an available HLA-identical donor. However, performing haploidentical-HSCT without any graft manipulation has historically been associated with a high risk of acute and chronic graft-versus-host disease (GVHD). T cell depletion reduces the risk of GVHD, but leads to delayed immune reconstitution, predisposing to serious infection and leukemia relapse due to the lack of a T-cell mediated graft-versus-leukemia (GvL). To address these challenges, we have infused mature BPX-501 T cells (donor peripheral lymphocytes which have been modified with the iCasp9 suicide gene) after αβ T-cell depleted haplo HSCT to facilitate immune reconstitution and GvL effect. BPX-501 T-cells are genetically modified with the iCasp9 suicide safety switch and a truncated CD19 marker. In the event of GvHD, the switch is activated by an infusion of the drug rimiducid (AP1903) resulting in rapid T cell apoptosis and GvHD reversal. CD3+/CD19+ T-cells can be tracked by flow cytometry.
Aims
This study was performed to evaluate both safety and efficacy of BPX-501 T cell infusion post αβ T-cell depleted haplo HSCT in pediatric patients with high risk ALL and AML in CR1 and CR2.
Methods
: A prospective Phase I-II study enrolling children with hematopoietic disorder who lack a matched donor. 38 patients have been enrolled and treated with αβ TCR depleted haplo HSCT after a myeloablative preparative regimen followed by BPX-T cell infusion to date; of them, 24 had ALL and 14 AML (21% CR1, 79% CR2). Median follow-up is 11 months (range 3-24).
Results
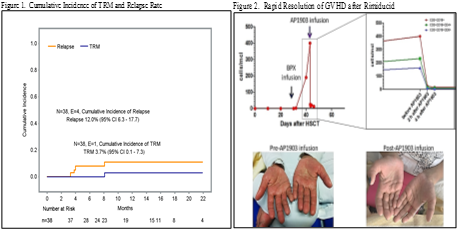
All patients engrafted and no secondary graft failure was recorded. Median time to neutrophil and platelet recovery was 16 days (range 8-33) and 11 days (range 7-19), respectively. With a median follow-up of 11 months (range 3-24 months), the cumulative incidence of NRM and relapse was 3.7% and 12.0%, respectively, while the disease-free survival probability was 84.2% (Fig 1). All aGVHD resolved (5 Grade I skin, 5 Grade II skin, 2 Grade III GI). One child received rimiducid to treat steroid-resistant grade II skin with complete resolution in 24 hours (Fig 2). There were 3 cases of chronic GvHD, 2 were mild; 1 severe and fatal in a patient whose donor had VZV reactivation during mobilization. CD3+ T cells reached 500 cells/μl by day 90, with normalized CD4/CD8 T cell ratio by day 180.
Conclusion
Engraftment was brisk and T cell recovery normalized by 6 months. Overall incidence of severe aGVHD was low and the safety switch was successfully activated with rimiducid infusion. Cumulative incidence of NRM compares favorably to historic controls at the lead center, where a value of of 2.4% for matched related donors (MR), 11.8% for matched unrelated donors (MUD) and 5% for αβ T cell depletion haplo HSCT (Haplo αβ) without BPX-501 infusion was recorded (Bertaina, 2015 ASH). The cumulative incidence of relapse was 12.0% for BPX-501, 32.3% for MR, 22.2% for MUDs and 21.9% Haplo-αβ. Disease-free survival in the BPX-501 treated patients was 84.2% compared to 65.4% for MR, 66.1% for MUDs and 73.1% for Haplo-αβ. However, length of follow-up on the control cohorts differed from that of BPX-501 treated patients. These data suggest that BPX-501 T cells modified with the iCasp9 safety switch, infused after selective αβ T-cell depletion, are safe and result in a rapid immune reconstitution and a potentially stronger GvL effect in children with high-risk leukemia who lack a matched donor.
Session topic: 22. Stem cell transplantation - Clinical
Keyword(s): Suicide gene, Pediatric, Leukemia, Haploidentical stem cell transplantation
Abstract: S495
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 17:00 - 17:15
Location: Room N103
Background
HLA-haploidentical allogeneic hematopoietic stem cell transplant (haplo-HSCT) offers an option for children with acute leukemia in need of a transplant and lacking an available HLA-identical donor. However, performing haploidentical-HSCT without any graft manipulation has historically been associated with a high risk of acute and chronic graft-versus-host disease (GVHD). T cell depletion reduces the risk of GVHD, but leads to delayed immune reconstitution, predisposing to serious infection and leukemia relapse due to the lack of a T-cell mediated graft-versus-leukemia (GvL). To address these challenges, we have infused mature BPX-501 T cells (donor peripheral lymphocytes which have been modified with the iCasp9 suicide gene) after αβ T-cell depleted haplo HSCT to facilitate immune reconstitution and GvL effect. BPX-501 T-cells are genetically modified with the iCasp9 suicide safety switch and a truncated CD19 marker. In the event of GvHD, the switch is activated by an infusion of the drug rimiducid (AP1903) resulting in rapid T cell apoptosis and GvHD reversal. CD3+/CD19+ T-cells can be tracked by flow cytometry.
Aims
This study was performed to evaluate both safety and efficacy of BPX-501 T cell infusion post αβ T-cell depleted haplo HSCT in pediatric patients with high risk ALL and AML in CR1 and CR2.
Methods
: A prospective Phase I-II study enrolling children with hematopoietic disorder who lack a matched donor. 38 patients have been enrolled and treated with αβ TCR depleted haplo HSCT after a myeloablative preparative regimen followed by BPX-T cell infusion to date; of them, 24 had ALL and 14 AML (21% CR1, 79% CR2). Median follow-up is 11 months (range 3-24).
Results
All patients engrafted and no secondary graft failure was recorded. Median time to neutrophil and platelet recovery was 16 days (range 8-33) and 11 days (range 7-19), respectively. With a median follow-up of 11 months (range 3-24 months), the cumulative incidence of NRM and relapse was 3.7% and 12.0%, respectively, while the disease-free survival probability was 84.2% (Fig 1). All aGVHD resolved (5 Grade I skin, 5 Grade II skin, 2 Grade III GI). One child received rimiducid to treat steroid-resistant grade II skin with complete resolution in 24 hours (Fig 2). There were 3 cases of chronic GvHD, 2 were mild; 1 severe and fatal in a patient whose donor had VZV reactivation during mobilization. CD3+ T cells reached 500 cells/μl by day 90, with normalized CD4/CD8 T cell ratio by day 180.

Conclusion
Engraftment was brisk and T cell recovery normalized by 6 months. Overall incidence of severe aGVHD was low and the safety switch was successfully activated with rimiducid infusion. Cumulative incidence of NRM compares favorably to historic controls at the lead center, where a value of of 2.4% for matched related donors (MR), 11.8% for matched unrelated donors (MUD) and 5% for αβ T cell depletion haplo HSCT (Haplo αβ) without BPX-501 infusion was recorded (Bertaina, 2015 ASH). The cumulative incidence of relapse was 12.0% for BPX-501, 32.3% for MR, 22.2% for MUDs and 21.9% Haplo-αβ. Disease-free survival in the BPX-501 treated patients was 84.2% compared to 65.4% for MR, 66.1% for MUDs and 73.1% for Haplo-αβ. However, length of follow-up on the control cohorts differed from that of BPX-501 treated patients. These data suggest that BPX-501 T cells modified with the iCasp9 safety switch, infused after selective αβ T-cell depletion, are safe and result in a rapid immune reconstitution and a potentially stronger GvL effect in children with high-risk leukemia who lack a matched donor.
Session topic: 22. Stem cell transplantation - Clinical
Keyword(s): Suicide gene, Pediatric, Leukemia, Haploidentical stem cell transplantation


