
Contributions
Abstract: S477
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:15 - 16:30
Location: Hall E
Background
CTL019 is an investigational therapy whereby autologous T cells are genetically engineered with a chimeric antigen receptor (CAR) to identify and eliminate CD19-expressing malignant B cells. Data from 2 phase 2 studies (ELIANA; NCT02435849 and ENSIGN; NCT02228096) in pediatric and young adult R/R B-cell ALL were pooled to evaluate cellular kinetics of CTL019.
Aims
We report cellular kinetics, humoral immunogenicity, AUC0-28d (exposure)–response analysis and impact of intrinsic/extrinsic and manufacturing factors on CTL019 expansion.
Methods
Cellular kinetic parameters of CTL019 post infusion were derived using traditional pharmacokinetic principles and reported by response category (complete response [CR]/CR with incomplete blood count recovery [CRi] vs no response [NR]) using 2 assays of peripheral blood cells: qPCR and flow cytometry. AUC0-28d–response relationships were evaluated by logistic regression. Relationships between manufacturing specifications, therapies for cytokine release syndrome (CRS) management, and anti-CAR19 antibodies on cellular kinetics were explored using summary statistics and graphical- and model-based analyses.
Results
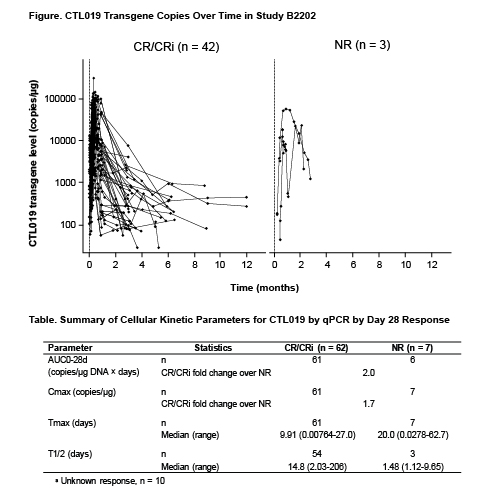
Data from 79 pts (ELIANA, n=50; ENSIGN, n=29) were pooled for analysis. Using qPCR, pts with CR/CRi (n=62) had ≈ 2-fold higher CTL019 expansion than pts with NR (n=7) (Cmax, 73.5% higher geometric [geo] mean; AUC0-28d, 104% higher geo mean; Table). Pts with NR had delayed Tmax compared with pts with CR/CRi (20 vs 10 days). Intrinsic pt factors including baseline cytogenetics, disease characteristics, and disease status did not appear to affect Cmax or AUC0-28d with the exception that pts with a higher tumor burden at enrollment generally had higher expansion, based on box plots and summary statistics. Extrinsic factors (prior lines of therapy, stem cell transplant) and parameters related to the manufactured product (% T cells, transduction efficiency, cell viability, total cell count), did not appear to impact cellular kinetics, based on graphical analysis. AUC0-28d increased with presence and severity of CRS. Pts who received anti-cytokine agents for grade 3/4 CRS also had higher expansion. CR/CRi pts treated with tocilizumab and steroids (n=17) had 89% higher AUC0-28d than CR pts who did not receive tocilizumab and steroids (n=45). Experience is limited in NR pts with (n=4) and without (n=4) tocilizumab. Moderate correlation was observed between transgene levels and CAR surface expression in peripheral blood (r2=0.592) by qPCR and flow cytometry, respectively, when matched by time points from the cellular kinetic profile. Slower B-cell recovery was observed in pts with AUC0-28d above the median. Post-dose anti-CAR19 antibody responses were determined from the fold change of anti-CAR19 antibodies above the baseline pre-dose value. Pts with treatment-induced or boosted anti-CAR19 antibody responses generally had lower expansion, based on box plots, compared with pts with treatment-unaffected anti-CAR19 antibody responses, although AUC0-28d was variable. The boosted levels of anti-CAR19 did not impact clinical response or relapse.
Conclusion
There was increased expansion of CTL019 in pts with higher tumor burden at enrollment, which correlated with higher CRS grade. There was no relationship between dose and expansion, supporting the wide dose range used. Expansion was not attenuated by tocilizumab or steroids, indicating therapies for CRS do not abrogate CTL019 proliferation. Cellular kinetics are important to understand the determinants of tumor response with CAR T-cell therapy.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Pharmacokinetic, Acute lymphoblastic leukemia
Abstract: S477
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:15 - 16:30
Location: Hall E
Background
CTL019 is an investigational therapy whereby autologous T cells are genetically engineered with a chimeric antigen receptor (CAR) to identify and eliminate CD19-expressing malignant B cells. Data from 2 phase 2 studies (ELIANA; NCT02435849 and ENSIGN; NCT02228096) in pediatric and young adult R/R B-cell ALL were pooled to evaluate cellular kinetics of CTL019.
Aims
We report cellular kinetics, humoral immunogenicity, AUC0-28d (exposure)–response analysis and impact of intrinsic/extrinsic and manufacturing factors on CTL019 expansion.
Methods
Cellular kinetic parameters of CTL019 post infusion were derived using traditional pharmacokinetic principles and reported by response category (complete response [CR]/CR with incomplete blood count recovery [CRi] vs no response [NR]) using 2 assays of peripheral blood cells: qPCR and flow cytometry. AUC0-28d–response relationships were evaluated by logistic regression. Relationships between manufacturing specifications, therapies for cytokine release syndrome (CRS) management, and anti-CAR19 antibodies on cellular kinetics were explored using summary statistics and graphical- and model-based analyses.
Results
Data from 79 pts (ELIANA, n=50; ENSIGN, n=29) were pooled for analysis. Using qPCR, pts with CR/CRi (n=62) had ≈ 2-fold higher CTL019 expansion than pts with NR (n=7) (Cmax, 73.5% higher geometric [geo] mean; AUC0-28d, 104% higher geo mean; Table). Pts with NR had delayed Tmax compared with pts with CR/CRi (20 vs 10 days). Intrinsic pt factors including baseline cytogenetics, disease characteristics, and disease status did not appear to affect Cmax or AUC0-28d with the exception that pts with a higher tumor burden at enrollment generally had higher expansion, based on box plots and summary statistics. Extrinsic factors (prior lines of therapy, stem cell transplant) and parameters related to the manufactured product (% T cells, transduction efficiency, cell viability, total cell count), did not appear to impact cellular kinetics, based on graphical analysis. AUC0-28d increased with presence and severity of CRS. Pts who received anti-cytokine agents for grade 3/4 CRS also had higher expansion. CR/CRi pts treated with tocilizumab and steroids (n=17) had 89% higher AUC0-28d than CR pts who did not receive tocilizumab and steroids (n=45). Experience is limited in NR pts with (n=4) and without (n=4) tocilizumab. Moderate correlation was observed between transgene levels and CAR surface expression in peripheral blood (r2=0.592) by qPCR and flow cytometry, respectively, when matched by time points from the cellular kinetic profile. Slower B-cell recovery was observed in pts with AUC0-28d above the median. Post-dose anti-CAR19 antibody responses were determined from the fold change of anti-CAR19 antibodies above the baseline pre-dose value. Pts with treatment-induced or boosted anti-CAR19 antibody responses generally had lower expansion, based on box plots, compared with pts with treatment-unaffected anti-CAR19 antibody responses, although AUC0-28d was variable. The boosted levels of anti-CAR19 did not impact clinical response or relapse.

Conclusion
There was increased expansion of CTL019 in pts with higher tumor burden at enrollment, which correlated with higher CRS grade. There was no relationship between dose and expansion, supporting the wide dose range used. Expansion was not attenuated by tocilizumab or steroids, indicating therapies for CRS do not abrogate CTL019 proliferation. Cellular kinetics are important to understand the determinants of tumor response with CAR T-cell therapy.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Pharmacokinetic, Acute lymphoblastic leukemia


