Abstract: S474
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:45 - 17:00
Location: Hall D
Background
Blocking PD-1/PD-L1 pathways enhances anti-leukemia responses in murine AML (Zhang et al, Blood 2009). PD-1 positive CD8 T-cells are increased in bone marrow (BM) of pts with AML (Daver et al, AACR 2016). AZA up-regulates PD-1 and interferon-gamma signaling in AML and the up-regulation of PD-1 has been associated with emergence of resistance to AZA (Yang et al., Leukemia 2013).
Aims
To assess the best response to Aza+Nivo at the end of 3 courses of combination therapy.
Methods
Pts were eligible if they had AML and failed prior therapy, had adequate performance status (ECOG ≤ 2), and organ function. The first six pts received AZA 75mg/m2 Days 1-7 with nivolumab 3mg/kg on Day 1 and 14. Courses were repeated every 4-5 weeks indefinitely. Only one of six pts had a dose limiting toxicity (grade 3 pneumonitis) and this dose was RP2D. 60 additional pts have been treated at the RP2D.
Results
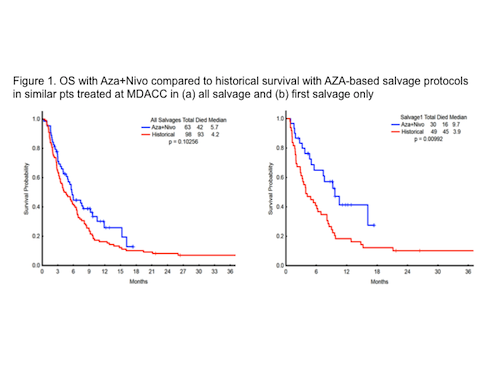
66 pts with a median age of 71 years (range, 44-90), secondary AML (39%), poor risk cytogenetics (35%), median number of prior regimens 2 (range, 1-7) have been enrolled. All 66 pts had baseline next generation sequencing: TP53 (n=14), DNMT3A (n=12), ASXL1 (n=10), TET2 (N=9), and RAS (n=9), IDH2 (n=9), IDH1 (n=6), CEBPA (n=7). 63 pts are evaluable for response: 14 (22%) achieved complete remission (CR)/complete remission with insufficient recovery of counts (CRi) (3 CR, 11 CRi), 7 (11%) had hematologic improvement (HI), 13 (21%) had ≥50% BM blast reduction, 5 pts (8%) had stable disease >6 months, and 24 (38%) had progression. 3 pts are too early for response assessment (<3 courses). The median number of courses to CR/CRi/HI was 2 (range, 1-4+). The med OS among the CR/CRi patients was 15.3 months (range, 2.29-17.25+), HI pts was 9.7 months (range, 4.67-17.45+), and NR was 5.0 months (range, 0.29-16.16). The 4- and 8-week mortality were 5% and 11%, respectively. The median OS for the 63 evaluable pts on Aza+Nivo compares favorably to historical median OS with AZA-based salvage protocols in similar pts treated at MDACC (P=0.10) (Fig 1A and Fig 1B)
Grade 3/4 and Grade 2 immune toxicities were observed in 8 (12%) and 7 (11%) pts, respectively. The most common Grade 3/4 AEs on treatment included pneumonitis, colitis, nephritis, skin rash, and hypophysitis. One pt died from grade 4 pneumonitis/epiglottitis. In the remaining 14 cases the toxicities responded rapidly to steroids and 13 of these pts were successfully rechallenged with nivolumab. Time to onset of toxicities ranged from 4 days to 3.5 months.
Multicolor flow-cytometry studies and Mass-cytometry (CyTOF) studies are conducted by the Immunotherapy Platform on baseline and on-treatment BM aspirate (end of cycle 1, 2, 4, 8). Baseline and end of cycle (EOC) 1 and 2 BM was evaluated in 6 responders and 19 non-responders. Pts who achieved a response had a baseline higher live total CD3 (P=0.10), CD8+ T-cells (P=0.02), and lower live CD4+Foxp3+PD1+ T-regulatory (T-reg) cells (P=0.01) infiltrate in BM. Patients who had a response had progressive increase in BM CD3+ cells and BM CD8+ cells, with increased ICOS (activation) marker on BM CD4-effector cells at EOC 1 and EOC 2 as compared to those who had no response. The CTLA4 on CD8 T-cells went up in both responders and non-responders after PD1 based therapy.
Conclusion
Full dose AZA and nivolumab are tolerable and produce an encouraging response rate with durable responses in relapsed AML with poor risk features. Immune mediated toxicities occur and may be adequately managed with early recognition and systemic steroids. Up-regulation of CTLA4 may be a mechanism of resistance to PD1 based therapies in AML and suggest role for combination therapy.
Session topic: 4. Acute myeloid leukemia - Clinical





