
Contributions
Abstract: S456
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:00 - 16:15
Location: Hall A
Background
Elotuzumab is an immunostimulatory monoclonal antibody that targets SLAMF7, a glycoprotein highly expressed on multiple myeloma (MM) cells and natural killer cells. Elotuzumab exerts a dual effect, directly activating natural killer cells and mediating MM cell death via antibody-dependent cell-mediated cytotoxicity. In a 3-year follow-up of ELOQUENT-2 (NCT01239797), elotuzumab plus lenalidomide/dexamethasone (ELd) demonstrated a sustained 27% reduction in the risk of disease progression/death and an overall survival (OS) trend towards benefit compared with lenalidomide/dexamethasone (Ld) alone in patients with relapsed/refractory (RR) MM (Dimopoulos et al, ASH 2015).
Aims
To evaluate the long-term efficacy and safety of ELd following extended 4-year follow-up (median 46 months).
Methods
RRMM patients with 1–3 prior lines of therapy randomized 1:1 received ELd or Ld in 28-day cycles until disease progression/unacceptable toxicity or consent withdrawal. Co-primary endpoints were progression-free survival (PFS) and overall response rate (ORR); OS was a secondary endpoint (analysis not prespecified for this data cut) and safety an exploratory endpoint. Written informed consent was obtained for all patients.
Results
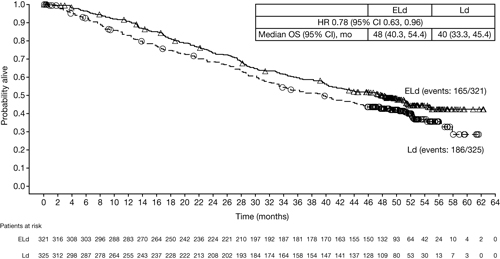
In total, 646 RRMM patients were randomized: 321 to ELd and 325 to Ld. At 4-year follow-up (data cut-off: Oct 18, 2016), nearly twice as many patients remained on ELd therapy vs Ld (17% vs 9%). With the extended follow-up, ELd demonstrated a sustained relative improvement of 50% in PFS rates vs Ld (21% vs 14%) and maintained reduction in the risk of progression/death of 29% for ELd vs Ld (all randomized patients: HR 0.71; 95% CI 0.59, 0.86). Patients with ≥ very good partial response (VGPR) (ELd 112 [35%] vs Ld 95 [29%]) had the greatest reduction (35%) in risk of progression/death (HR 0.65; 95% CI 0.46, 0.94). ORR was greater with ELd vs Ld (79% vs 66%) and the duration of response benefit was maintained over time (HR 0.77; 95% CI 0.62, 0.95). Early separation of the Kaplan–Meier survival curves, which remained consistently separated over time, supports a sustained OS benefit in favor of ELd vs Ld (Figure). Grade 3–4 adverse events (AEs) in ≥5% of patients were generally comparable between ELd and Ld arms—vascular diseases (10% vs 8%; mostly venous-related), second primary malignancies (SPMs; 9% vs 6%) and cardiac disorders (5% vs 8%); the exception was a slightly higher incidence of infection with ELd (33% vs 26%). Overall rate (any grade) of infection (84% vs 75%) and SPMs (17% vs 11%) was also higher for ELd vs Ld. However, exposure to ELd was longer than to Ld (median [Q1, Q3] treatment cycles: 19 [9, 42] vs 14 [6, 25]). Disease progression and infection were major causes of mortality in both arms; however, fewer deaths were reported with ELd vs Ld (165 vs 186).
Conclusion
At 4 years, ELd has the longest median follow-up of an immuno-oncology agent in MM. The data continue to show that adding elotuzumab to Ld results in durable long-term responses, clinically relevant improvement in PFS, sustained reduction in risk of progression/death, and a survival trend in favor of ELd. Overall, these data continue to support the durable efficacy of ELd. Updated safety and tolerability, including rate of SPMs, was consistent with previous findings despite longer exposure, with minimal incremental AEs compared with Ld therapy.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Survival, Multiple Myeloma, Immunotherapy, Follow-up
Abstract: S456
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:00 - 16:15
Location: Hall A
Background
Elotuzumab is an immunostimulatory monoclonal antibody that targets SLAMF7, a glycoprotein highly expressed on multiple myeloma (MM) cells and natural killer cells. Elotuzumab exerts a dual effect, directly activating natural killer cells and mediating MM cell death via antibody-dependent cell-mediated cytotoxicity. In a 3-year follow-up of ELOQUENT-2 (NCT01239797), elotuzumab plus lenalidomide/dexamethasone (ELd) demonstrated a sustained 27% reduction in the risk of disease progression/death and an overall survival (OS) trend towards benefit compared with lenalidomide/dexamethasone (Ld) alone in patients with relapsed/refractory (RR) MM (Dimopoulos et al, ASH 2015).
Aims
To evaluate the long-term efficacy and safety of ELd following extended 4-year follow-up (median 46 months).
Methods
RRMM patients with 1–3 prior lines of therapy randomized 1:1 received ELd or Ld in 28-day cycles until disease progression/unacceptable toxicity or consent withdrawal. Co-primary endpoints were progression-free survival (PFS) and overall response rate (ORR); OS was a secondary endpoint (analysis not prespecified for this data cut) and safety an exploratory endpoint. Written informed consent was obtained for all patients.
Results
In total, 646 RRMM patients were randomized: 321 to ELd and 325 to Ld. At 4-year follow-up (data cut-off: Oct 18, 2016), nearly twice as many patients remained on ELd therapy vs Ld (17% vs 9%). With the extended follow-up, ELd demonstrated a sustained relative improvement of 50% in PFS rates vs Ld (21% vs 14%) and maintained reduction in the risk of progression/death of 29% for ELd vs Ld (all randomized patients: HR 0.71; 95% CI 0.59, 0.86). Patients with ≥ very good partial response (VGPR) (ELd 112 [35%] vs Ld 95 [29%]) had the greatest reduction (35%) in risk of progression/death (HR 0.65; 95% CI 0.46, 0.94). ORR was greater with ELd vs Ld (79% vs 66%) and the duration of response benefit was maintained over time (HR 0.77; 95% CI 0.62, 0.95). Early separation of the Kaplan–Meier survival curves, which remained consistently separated over time, supports a sustained OS benefit in favor of ELd vs Ld (Figure). Grade 3–4 adverse events (AEs) in ≥5% of patients were generally comparable between ELd and Ld arms—vascular diseases (10% vs 8%; mostly venous-related), second primary malignancies (SPMs; 9% vs 6%) and cardiac disorders (5% vs 8%); the exception was a slightly higher incidence of infection with ELd (33% vs 26%). Overall rate (any grade) of infection (84% vs 75%) and SPMs (17% vs 11%) was also higher for ELd vs Ld. However, exposure to ELd was longer than to Ld (median [Q1, Q3] treatment cycles: 19 [9, 42] vs 14 [6, 25]). Disease progression and infection were major causes of mortality in both arms; however, fewer deaths were reported with ELd vs Ld (165 vs 186).

Conclusion
At 4 years, ELd has the longest median follow-up of an immuno-oncology agent in MM. The data continue to show that adding elotuzumab to Ld results in durable long-term responses, clinically relevant improvement in PFS, sustained reduction in risk of progression/death, and a survival trend in favor of ELd. Overall, these data continue to support the durable efficacy of ELd. Updated safety and tolerability, including rate of SPMs, was consistent with previous findings despite longer exposure, with minimal incremental AEs compared with Ld therapy.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Survival, Multiple Myeloma, Immunotherapy, Follow-up


