
Contributions
Abstract: S454
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 12:15 - 12:30
Location: Room N109
Background
Sickle cell-related pain crises (SCPCs) are a substantial cause of morbidity in patients with sickle cell disease (SCD). At a dose of 5.0 mg/kg, the P‑selectin inhibitor crizanlizumab, delivered intravenously every 4 weeks after loading, was shown to significantly reduce the frequency of SCPC events and was well tolerated in the 52-week SUSTAIN study (Ataga KI et al. N Engl J Med 2017;376:429–439).
Aims
This post-hoc analysis evaluated patients who did not experience a SCPC for the duration of the trial.
Methods
SUSTAIN was a randomized, double-blind, placebo-controlled, Phase II study (NCT01895361). Patients aged 16–65 years with SCD (including HbSS, HbSC, HbSβ0–thalassaemia, and HbSβ+–thalassaemia genotypes) and 2–10 SCPC events in the previous 12 months were included. Concomitant use of hydroxyurea (HU) was permitted if the patient had been using it for ≥6 months and at a stable dose for ≥3 months. Patients were randomized 1:1:1 to receive intravenous crizanlizumab 5.0 mg/kg, 2.5 mg/kg or placebo. Loading doses were administered on days 1 and 15, followed by routine treatment every 4 weeks to week 50, with the final assessment visit at week 52. Descriptive statistics were used to summarize the frequency of patients who were SCPC event-free for the duration of the study, based on the intent-to-treat (ITT) population overall and by prior SCPC events, SCD genotype and HU use at baseline.
Results
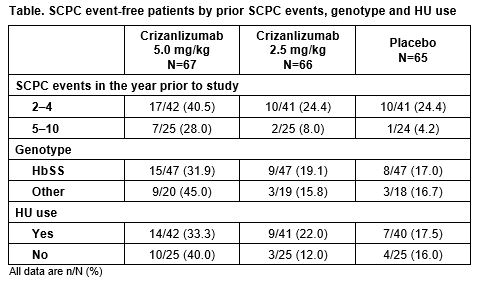
Among the 198 patients included in the study (ITT population), 62.6% and 37.4% had experienced 2–4 and 5–10 SCPC events in the previous year, respectively, and 62.1% were taking HU at baseline. HbSS was the most common genotype (71.2%; HbSC: 16.2%, HbSβ0–thalassemia: 6.1%, HbSβ+–thalassemia: 5.1%, other: 1.5%). Overall, more patients in the crizanlizumab 5.0 mg/kg group (n=24/67; 35.8%) were SCPC event-free than in the 2.5 mg/kg (n=12/66; 18.2%) and placebo (n=11/65; 16.9%) groups. In each of the prior SCPC events, SCD genotype and HU use subgroups, a greater proportion of patients treated with crizanlizumab 5.0 mg/kg were SCPC event-free compared with those in the crizanlizumab 2.5 mg/kg or placebo arms (Table). In subpopulations considered to be at increased risk of experiencing a SCPC (patients with 5–10 SCPC events in the previous year and/or with the homozygous HbSS genotype), a higher proportion of patients treated with crizanlizumab 5.0 mg/kg were SCPC event-free compared with those in the placebo arm (28.0% vs 4.2% and 31.9% vs 17.0%, respectively). Additionally, 33.3% of patients who were taking HU and treated with crizanlizumab 5.0 mg/kg were SCPC event-free during the study, compared with 17.5% in the placebo arm, possibly suggesting an additive effect.
Conclusion
Treatment with crizanlizumab 5.0 mg/kg appears to increase the likelihood of adult patients with SCD being SCPC event-free while on treatment, even in high-risk subpopulations. Crizanlizumab 5.0 mg/kg was also effective in those who had experienced at least two SCPCs in the previous year despite taking HU, suggesting that this dose is effective as a disease-modifying agent that meets an unmet medical need.
Session topic: 25. Sickle cell disease
Keyword(s): sickle cell disease, Sickle cell adhesion, P-selectin
Abstract: S454
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 12:15 - 12:30
Location: Room N109
Background
Sickle cell-related pain crises (SCPCs) are a substantial cause of morbidity in patients with sickle cell disease (SCD). At a dose of 5.0 mg/kg, the P‑selectin inhibitor crizanlizumab, delivered intravenously every 4 weeks after loading, was shown to significantly reduce the frequency of SCPC events and was well tolerated in the 52-week SUSTAIN study (Ataga KI et al. N Engl J Med 2017;376:429–439).
Aims
This post-hoc analysis evaluated patients who did not experience a SCPC for the duration of the trial.
Methods
SUSTAIN was a randomized, double-blind, placebo-controlled, Phase II study (NCT01895361). Patients aged 16–65 years with SCD (including HbSS, HbSC, HbSβ0–thalassaemia, and HbSβ+–thalassaemia genotypes) and 2–10 SCPC events in the previous 12 months were included. Concomitant use of hydroxyurea (HU) was permitted if the patient had been using it for ≥6 months and at a stable dose for ≥3 months. Patients were randomized 1:1:1 to receive intravenous crizanlizumab 5.0 mg/kg, 2.5 mg/kg or placebo. Loading doses were administered on days 1 and 15, followed by routine treatment every 4 weeks to week 50, with the final assessment visit at week 52. Descriptive statistics were used to summarize the frequency of patients who were SCPC event-free for the duration of the study, based on the intent-to-treat (ITT) population overall and by prior SCPC events, SCD genotype and HU use at baseline.
Results
Among the 198 patients included in the study (ITT population), 62.6% and 37.4% had experienced 2–4 and 5–10 SCPC events in the previous year, respectively, and 62.1% were taking HU at baseline. HbSS was the most common genotype (71.2%; HbSC: 16.2%, HbSβ0–thalassemia: 6.1%, HbSβ+–thalassemia: 5.1%, other: 1.5%). Overall, more patients in the crizanlizumab 5.0 mg/kg group (n=24/67; 35.8%) were SCPC event-free than in the 2.5 mg/kg (n=12/66; 18.2%) and placebo (n=11/65; 16.9%) groups. In each of the prior SCPC events, SCD genotype and HU use subgroups, a greater proportion of patients treated with crizanlizumab 5.0 mg/kg were SCPC event-free compared with those in the crizanlizumab 2.5 mg/kg or placebo arms (Table). In subpopulations considered to be at increased risk of experiencing a SCPC (patients with 5–10 SCPC events in the previous year and/or with the homozygous HbSS genotype), a higher proportion of patients treated with crizanlizumab 5.0 mg/kg were SCPC event-free compared with those in the placebo arm (28.0% vs 4.2% and 31.9% vs 17.0%, respectively). Additionally, 33.3% of patients who were taking HU and treated with crizanlizumab 5.0 mg/kg were SCPC event-free during the study, compared with 17.5% in the placebo arm, possibly suggesting an additive effect.

Conclusion
Treatment with crizanlizumab 5.0 mg/kg appears to increase the likelihood of adult patients with SCD being SCPC event-free while on treatment, even in high-risk subpopulations. Crizanlizumab 5.0 mg/kg was also effective in those who had experienced at least two SCPCs in the previous year despite taking HU, suggesting that this dose is effective as a disease-modifying agent that meets an unmet medical need.
Session topic: 25. Sickle cell disease
Keyword(s): sickle cell disease, Sickle cell adhesion, P-selectin


