THERAPEUTIC TARGETING OF ONCOGENIC MYB ACTIVITY IN T-ALL
(Abstract release date: 05/18/17)
EHA Library. Pieters T. 06/24/17; 181725; S438

Dr. Tim Pieters
Contributions
Contributions
Abstract
Abstract: S438
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 12:00 - 12:15
Location: Room N105
Background
T-lineage acute lymphoblastic leukemia (T-ALL) is an aggressive hematologic malignancy that accounts for 10%–15% of pediatric and 25% of adult ALL cases. The prognosis of T-ALL has gradually improved, however, the outcome of T-ALL patients with primary resistant or relapsed leukemia remains poor. Thus, further advances in the treatment of T-ALL require the development of effective and highly specific molecularly targeted antileukemic drugs.
The proto-oncogene MYB (encodes c-MYB) is aberrantly activated in a subset of T-ALL patients through T-cell receptor driven translocations or genomic duplications of the MYB locus itself. Recently, a new genetic mechanism for the generation of oncogenic super-enhancers in malignant T cells was identified, and suggests a general role for MYB in the regulation of T-cell specific super-enhancer activity.
Aims
We want to identify the role of enhanced MYB activity in super-enhancer driven oncogenic transcription in the context of malignant T-cell development and investigate the in vivo role of cMyb in the initiation and maintenance of T-ALL.
Methods
To evaluate if cMyb could act as a bona fide oncogene in the pathogenesis of T-ALL, we developed a conditional R26-driven cMyb overexpression mouse model. For this, we used a targeting vector that contained a floxed stop cassette followed by the cMyb gene and a EGFP/luciferase reporter, which was subsequently targeted in mESCs using recombinase-mediated cassette exchange (RMCE).
Results
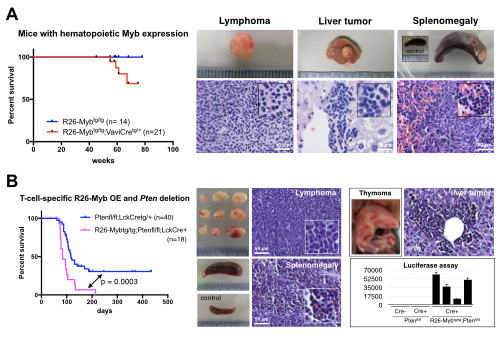
Here, we report a novel conditional Myb knockin mouse model (R26-Myb). To study the in vivo oncogenic capacity of Myb, we initially crossed this conditional Myb knockin model with VaviCre mice, in order to obtain hematopoeitic specific expression of Myb and the EGFP/luciferase from the ROSA26-promoter. Notably, Vav-iCretg/+ R26-Mybtg/tg mice developed T-cell lymphomas with a median latency of 77 weeks, suggesting that Myb can act as a bona fide oncogene in malignant T-cell transformation (Figure 1A).
Next, we crossed our Myb transgenic model with Pten conditional knockout mice, to allow comparative analysis of tumors with and without T-cell specific Myb expression. Genetic inactivation of Pten is frequently observed in human T-ALL, and T-cell specific deletion of Pten (using Lck-Cre) results in T-cell leukemia/lymphoma development with an average of 17 weeks. Using this strategy, we obtained mice that overexpress R26-driven cMyb and lack Pten in developing T-cells and found that cMyb expression synergizes with Pten deletion, resulting in fully penetrant and accelerated T-ALL formation (median survival of 84 days instead of 118; p =0.0003; Figure 1B).
Finally, we used this novel murine T-ALL model to identify new therapeutic strategies for MYB dependent T-ALL. Importantly, the tumor cells from the cMyb knockin mice are luciferase-positive and are therefore suitable for in vivo drug testing using bioluminescence. Using this model, we evaluated the in vivo anti-leukemic efficacy of a variety of small molecules and identified new drugs that impede Myb protein stability or Myb-mediated transactivation in Myb driven tumorigenesis.
Conclusion
We developed a novel Myb-driven T-ALL mouse model and could demonstrate a pathogenic role for cMYB in T-cell leukemia. In addition, the Myb-driven preclinical mouse model will open new avenues for therapeutic intervention in T-ALL.
Session topic: 1. Acute lymphoblastic leukemia - Biology
Keyword(s): C-myb, Acute lymphoblastic leukemia, Mouse model
Abstract: S438
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 12:00 - 12:15
Location: Room N105
Background
T-lineage acute lymphoblastic leukemia (T-ALL) is an aggressive hematologic malignancy that accounts for 10%–15% of pediatric and 25% of adult ALL cases. The prognosis of T-ALL has gradually improved, however, the outcome of T-ALL patients with primary resistant or relapsed leukemia remains poor. Thus, further advances in the treatment of T-ALL require the development of effective and highly specific molecularly targeted antileukemic drugs.
The proto-oncogene MYB (encodes c-MYB) is aberrantly activated in a subset of T-ALL patients through T-cell receptor driven translocations or genomic duplications of the MYB locus itself. Recently, a new genetic mechanism for the generation of oncogenic super-enhancers in malignant T cells was identified, and suggests a general role for MYB in the regulation of T-cell specific super-enhancer activity.
Aims
We want to identify the role of enhanced MYB activity in super-enhancer driven oncogenic transcription in the context of malignant T-cell development and investigate the in vivo role of cMyb in the initiation and maintenance of T-ALL.
Methods
To evaluate if cMyb could act as a bona fide oncogene in the pathogenesis of T-ALL, we developed a conditional R26-driven cMyb overexpression mouse model. For this, we used a targeting vector that contained a floxed stop cassette followed by the cMyb gene and a EGFP/luciferase reporter, which was subsequently targeted in mESCs using recombinase-mediated cassette exchange (RMCE).
Results
Here, we report a novel conditional Myb knockin mouse model (R26-Myb). To study the in vivo oncogenic capacity of Myb, we initially crossed this conditional Myb knockin model with VaviCre mice, in order to obtain hematopoeitic specific expression of Myb and the EGFP/luciferase from the ROSA26-promoter. Notably, Vav-iCretg/+ R26-Mybtg/tg mice developed T-cell lymphomas with a median latency of 77 weeks, suggesting that Myb can act as a bona fide oncogene in malignant T-cell transformation (Figure 1A).
Next, we crossed our Myb transgenic model with Pten conditional knockout mice, to allow comparative analysis of tumors with and without T-cell specific Myb expression. Genetic inactivation of Pten is frequently observed in human T-ALL, and T-cell specific deletion of Pten (using Lck-Cre) results in T-cell leukemia/lymphoma development with an average of 17 weeks. Using this strategy, we obtained mice that overexpress R26-driven cMyb and lack Pten in developing T-cells and found that cMyb expression synergizes with Pten deletion, resulting in fully penetrant and accelerated T-ALL formation (median survival of 84 days instead of 118; p =0.0003; Figure 1B).
Finally, we used this novel murine T-ALL model to identify new therapeutic strategies for MYB dependent T-ALL. Importantly, the tumor cells from the cMyb knockin mice are luciferase-positive and are therefore suitable for in vivo drug testing using bioluminescence. Using this model, we evaluated the in vivo anti-leukemic efficacy of a variety of small molecules and identified new drugs that impede Myb protein stability or Myb-mediated transactivation in Myb driven tumorigenesis.

Conclusion
We developed a novel Myb-driven T-ALL mouse model and could demonstrate a pathogenic role for cMYB in T-cell leukemia. In addition, the Myb-driven preclinical mouse model will open new avenues for therapeutic intervention in T-ALL.
Session topic: 1. Acute lymphoblastic leukemia - Biology
Keyword(s): C-myb, Acute lymphoblastic leukemia, Mouse model
{{ help_message }}
{{filter}}


