FINAL SAFETY AND EFFICACY RESULTS FROM THE EXTEND STUDY: TREATMENT WITH ELTROMBOPAG (EPAG) IN ADULTS WITH CHRONIC IMMUNE THROMBOCYTOPENIA (CITP)
(Abstract release date: 05/19/16)
EHA Library. Smith L. 06/11/16; 135273; S517

Lori Smith
Contributions
Contributions
Abstract
Abstract: S517
Type: Oral Presentation
Presentation during EHA21: On Saturday, June 11, 2016 from 16:15 - 16:30
Location: Room H4
Background
EPAG increased platelets and reduced bleeding in 6-week and 6-month placebo-controlled trials in previously treated cITP patients. Adult patients with cITP initially enrolled in 4 EPAG studies could continue treatment in the open-label extension study, EXTEND.
Aims
To present the final long-term safety and efficacy results from EXTEND (Jun 2006 to Jul 2015).
Methods
EPAG was started at 50 mg and titrated to 25–75 mg/day or less often, based on platelet counts. Maintenance dosing continued after minimization of concomitant ITP medication and optimization of EPAG dosing. Patients who received 2 years of EPAG and transitioned off due to commercial availability of EPAG were considered to have completed EXTEND, whether or not they continued treatment with commercial EPAG.
Results
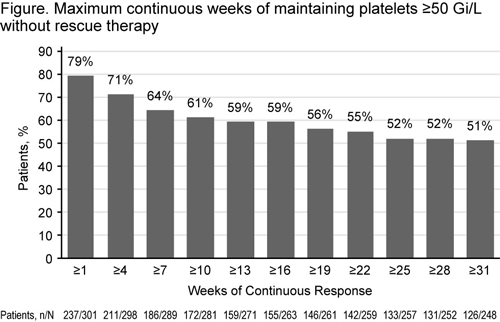
Of 302 patients enrolled, 67% were females and 38% were splenectomized; 45% (n=135) completed and 55% (n=167) withdrew. The most common reasons for withdrawal were adverse events (AEs; 14%), patient decision (13%), lack of efficacy (11%), and other (13%). The overall median duration of exposure was 2.4 years (range, 2 days to 8.8 years) and mean average daily dose was 50.2 (range, 1–75) mg/day. Median platelet counts increased to 50 Gi/L by Week 2. Overall, 86% (259/302) of patients achieved platelets 50 Gi/L in the absence of rescue therapy and 61% achieved platelets 50 Gi/L for 50% of on-treatment assessments; 126/248 (51%) patients maintained continuous platelet counts ≥50 Gi/L for at least 31 weeks (Figure). Incidence of bleeding symptoms (WHO grades 1–4) decreased from baseline (57%; 171/302) to 1 year (16%; 13/80). Proportionately more patients with platelet counts <10 Gi/L had grades 2–4 bleeding compared with those with higher platelet counts (59% vs ≤40%). Of 101 patients receiving concomitant ITP treatment at baseline, 34 stopped at least one ITP medication, and 39 had a sustained reduction or permanently stopped at least one ITP medication taken at baseline. The most frequently discontinued/reduced ITP medications were corticosteroids (n=34), danazol (n=5), azathioprine (n=4), and other (n=1). AEs occurred in 277 (92%) patients. Serious AEs (SAEs) occurred in 96 (32%) patients, and 24 (8%) patients had SAEs considered possibly drug-related. Drug-related SAEs occurring in 2 patients were cataracts (n=8), increased alanine aminotransferase (ALT) (n=4), deep vein thrombosis (n=2), increased aspartate aminotransferase (n=2), increased bilirubin (n=2), myocardial infarction (n=2), and pneumonia (n=2). AEs leading to withdrawal occurred in 41 (14%) patients. 28 (9%) patients experienced SAEs. The most frequent AEs leading to withdrawal were increased ALT (n=5), increased bilirubin (n=4), cataracts (n=4), and DVT (n=3). Nineteen patients (6%) reported thromboembolic events (TEEs), and 37 reported hepatobiliary laboratory abnormalities (HBLAs). Ocular-related events started on-therapy occurred in 80 (26%) patients; the most frequently occurring events were cataract (n=28; 9%) and conjunctivitis (n=12; 4%). At baseline, 192 patients had at least one cataract risk factor.
Conclusion
Sustained platelet increases and reduced bleeding symptoms were observed in EPAG-treated patients with cITP throughout the study. Concomitant ITP medications were reduced without requiring rescue medications. EPAG was well tolerated with exposures 6 years. Monitoring of HBLA and cataracts is appropriate, even with long-term treatment. Funding: Study (NCT00351468) is/remains sponsored by GlaxoSmithKline; EPAG is an asset of Novartis AG as of March 2, 2015.
Session topic: Platelet disorders 1
Keyword(s): Chronic ITP, Platelet count, Safety, Thrombopoietin (TPO)
Type: Oral Presentation
Presentation during EHA21: On Saturday, June 11, 2016 from 16:15 - 16:30
Location: Room H4
Background
EPAG increased platelets and reduced bleeding in 6-week and 6-month placebo-controlled trials in previously treated cITP patients. Adult patients with cITP initially enrolled in 4 EPAG studies could continue treatment in the open-label extension study, EXTEND.
Aims
To present the final long-term safety and efficacy results from EXTEND (Jun 2006 to Jul 2015).
Methods
EPAG was started at 50 mg and titrated to 25–75 mg/day or less often, based on platelet counts. Maintenance dosing continued after minimization of concomitant ITP medication and optimization of EPAG dosing. Patients who received 2 years of EPAG and transitioned off due to commercial availability of EPAG were considered to have completed EXTEND, whether or not they continued treatment with commercial EPAG.
Results
Of 302 patients enrolled, 67% were females and 38% were splenectomized; 45% (n=135) completed and 55% (n=167) withdrew. The most common reasons for withdrawal were adverse events (AEs; 14%), patient decision (13%), lack of efficacy (11%), and other (13%). The overall median duration of exposure was 2.4 years (range, 2 days to 8.8 years) and mean average daily dose was 50.2 (range, 1–75) mg/day. Median platelet counts increased to 50 Gi/L by Week 2. Overall, 86% (259/302) of patients achieved platelets 50 Gi/L in the absence of rescue therapy and 61% achieved platelets 50 Gi/L for 50% of on-treatment assessments; 126/248 (51%) patients maintained continuous platelet counts ≥50 Gi/L for at least 31 weeks (Figure). Incidence of bleeding symptoms (WHO grades 1–4) decreased from baseline (57%; 171/302) to 1 year (16%; 13/80). Proportionately more patients with platelet counts <10 Gi/L had grades 2–4 bleeding compared with those with higher platelet counts (59% vs ≤40%). Of 101 patients receiving concomitant ITP treatment at baseline, 34 stopped at least one ITP medication, and 39 had a sustained reduction or permanently stopped at least one ITP medication taken at baseline. The most frequently discontinued/reduced ITP medications were corticosteroids (n=34), danazol (n=5), azathioprine (n=4), and other (n=1). AEs occurred in 277 (92%) patients. Serious AEs (SAEs) occurred in 96 (32%) patients, and 24 (8%) patients had SAEs considered possibly drug-related. Drug-related SAEs occurring in 2 patients were cataracts (n=8), increased alanine aminotransferase (ALT) (n=4), deep vein thrombosis (n=2), increased aspartate aminotransferase (n=2), increased bilirubin (n=2), myocardial infarction (n=2), and pneumonia (n=2). AEs leading to withdrawal occurred in 41 (14%) patients. 28 (9%) patients experienced SAEs. The most frequent AEs leading to withdrawal were increased ALT (n=5), increased bilirubin (n=4), cataracts (n=4), and DVT (n=3). Nineteen patients (6%) reported thromboembolic events (TEEs), and 37 reported hepatobiliary laboratory abnormalities (HBLAs). Ocular-related events started on-therapy occurred in 80 (26%) patients; the most frequently occurring events were cataract (n=28; 9%) and conjunctivitis (n=12; 4%). At baseline, 192 patients had at least one cataract risk factor.
Conclusion
Sustained platelet increases and reduced bleeding symptoms were observed in EPAG-treated patients with cITP throughout the study. Concomitant ITP medications were reduced without requiring rescue medications. EPAG was well tolerated with exposures 6 years. Monitoring of HBLA and cataracts is appropriate, even with long-term treatment. Funding: Study (NCT00351468) is/remains sponsored by GlaxoSmithKline; EPAG is an asset of Novartis AG as of March 2, 2015.

Session topic: Platelet disorders 1
Keyword(s): Chronic ITP, Platelet count, Safety, Thrombopoietin (TPO)
Abstract: S517
Type: Oral Presentation
Presentation during EHA21: On Saturday, June 11, 2016 from 16:15 - 16:30
Location: Room H4
Background
EPAG increased platelets and reduced bleeding in 6-week and 6-month placebo-controlled trials in previously treated cITP patients. Adult patients with cITP initially enrolled in 4 EPAG studies could continue treatment in the open-label extension study, EXTEND.
Aims
To present the final long-term safety and efficacy results from EXTEND (Jun 2006 to Jul 2015).
Methods
EPAG was started at 50 mg and titrated to 25–75 mg/day or less often, based on platelet counts. Maintenance dosing continued after minimization of concomitant ITP medication and optimization of EPAG dosing. Patients who received 2 years of EPAG and transitioned off due to commercial availability of EPAG were considered to have completed EXTEND, whether or not they continued treatment with commercial EPAG.
Results
Of 302 patients enrolled, 67% were females and 38% were splenectomized; 45% (n=135) completed and 55% (n=167) withdrew. The most common reasons for withdrawal were adverse events (AEs; 14%), patient decision (13%), lack of efficacy (11%), and other (13%). The overall median duration of exposure was 2.4 years (range, 2 days to 8.8 years) and mean average daily dose was 50.2 (range, 1–75) mg/day. Median platelet counts increased to 50 Gi/L by Week 2. Overall, 86% (259/302) of patients achieved platelets 50 Gi/L in the absence of rescue therapy and 61% achieved platelets 50 Gi/L for 50% of on-treatment assessments; 126/248 (51%) patients maintained continuous platelet counts ≥50 Gi/L for at least 31 weeks (Figure). Incidence of bleeding symptoms (WHO grades 1–4) decreased from baseline (57%; 171/302) to 1 year (16%; 13/80). Proportionately more patients with platelet counts <10 Gi/L had grades 2–4 bleeding compared with those with higher platelet counts (59% vs ≤40%). Of 101 patients receiving concomitant ITP treatment at baseline, 34 stopped at least one ITP medication, and 39 had a sustained reduction or permanently stopped at least one ITP medication taken at baseline. The most frequently discontinued/reduced ITP medications were corticosteroids (n=34), danazol (n=5), azathioprine (n=4), and other (n=1). AEs occurred in 277 (92%) patients. Serious AEs (SAEs) occurred in 96 (32%) patients, and 24 (8%) patients had SAEs considered possibly drug-related. Drug-related SAEs occurring in 2 patients were cataracts (n=8), increased alanine aminotransferase (ALT) (n=4), deep vein thrombosis (n=2), increased aspartate aminotransferase (n=2), increased bilirubin (n=2), myocardial infarction (n=2), and pneumonia (n=2). AEs leading to withdrawal occurred in 41 (14%) patients. 28 (9%) patients experienced SAEs. The most frequent AEs leading to withdrawal were increased ALT (n=5), increased bilirubin (n=4), cataracts (n=4), and DVT (n=3). Nineteen patients (6%) reported thromboembolic events (TEEs), and 37 reported hepatobiliary laboratory abnormalities (HBLAs). Ocular-related events started on-therapy occurred in 80 (26%) patients; the most frequently occurring events were cataract (n=28; 9%) and conjunctivitis (n=12; 4%). At baseline, 192 patients had at least one cataract risk factor.
Conclusion
Sustained platelet increases and reduced bleeding symptoms were observed in EPAG-treated patients with cITP throughout the study. Concomitant ITP medications were reduced without requiring rescue medications. EPAG was well tolerated with exposures 6 years. Monitoring of HBLA and cataracts is appropriate, even with long-term treatment. Funding: Study (NCT00351468) is/remains sponsored by GlaxoSmithKline; EPAG is an asset of Novartis AG as of March 2, 2015.

Session topic: Platelet disorders 1
Keyword(s): Chronic ITP, Platelet count, Safety, Thrombopoietin (TPO)
Type: Oral Presentation
Presentation during EHA21: On Saturday, June 11, 2016 from 16:15 - 16:30
Location: Room H4
Background
EPAG increased platelets and reduced bleeding in 6-week and 6-month placebo-controlled trials in previously treated cITP patients. Adult patients with cITP initially enrolled in 4 EPAG studies could continue treatment in the open-label extension study, EXTEND.
Aims
To present the final long-term safety and efficacy results from EXTEND (Jun 2006 to Jul 2015).
Methods
EPAG was started at 50 mg and titrated to 25–75 mg/day or less often, based on platelet counts. Maintenance dosing continued after minimization of concomitant ITP medication and optimization of EPAG dosing. Patients who received 2 years of EPAG and transitioned off due to commercial availability of EPAG were considered to have completed EXTEND, whether or not they continued treatment with commercial EPAG.
Results
Of 302 patients enrolled, 67% were females and 38% were splenectomized; 45% (n=135) completed and 55% (n=167) withdrew. The most common reasons for withdrawal were adverse events (AEs; 14%), patient decision (13%), lack of efficacy (11%), and other (13%). The overall median duration of exposure was 2.4 years (range, 2 days to 8.8 years) and mean average daily dose was 50.2 (range, 1–75) mg/day. Median platelet counts increased to 50 Gi/L by Week 2. Overall, 86% (259/302) of patients achieved platelets 50 Gi/L in the absence of rescue therapy and 61% achieved platelets 50 Gi/L for 50% of on-treatment assessments; 126/248 (51%) patients maintained continuous platelet counts ≥50 Gi/L for at least 31 weeks (Figure). Incidence of bleeding symptoms (WHO grades 1–4) decreased from baseline (57%; 171/302) to 1 year (16%; 13/80). Proportionately more patients with platelet counts <10 Gi/L had grades 2–4 bleeding compared with those with higher platelet counts (59% vs ≤40%). Of 101 patients receiving concomitant ITP treatment at baseline, 34 stopped at least one ITP medication, and 39 had a sustained reduction or permanently stopped at least one ITP medication taken at baseline. The most frequently discontinued/reduced ITP medications were corticosteroids (n=34), danazol (n=5), azathioprine (n=4), and other (n=1). AEs occurred in 277 (92%) patients. Serious AEs (SAEs) occurred in 96 (32%) patients, and 24 (8%) patients had SAEs considered possibly drug-related. Drug-related SAEs occurring in 2 patients were cataracts (n=8), increased alanine aminotransferase (ALT) (n=4), deep vein thrombosis (n=2), increased aspartate aminotransferase (n=2), increased bilirubin (n=2), myocardial infarction (n=2), and pneumonia (n=2). AEs leading to withdrawal occurred in 41 (14%) patients. 28 (9%) patients experienced SAEs. The most frequent AEs leading to withdrawal were increased ALT (n=5), increased bilirubin (n=4), cataracts (n=4), and DVT (n=3). Nineteen patients (6%) reported thromboembolic events (TEEs), and 37 reported hepatobiliary laboratory abnormalities (HBLAs). Ocular-related events started on-therapy occurred in 80 (26%) patients; the most frequently occurring events were cataract (n=28; 9%) and conjunctivitis (n=12; 4%). At baseline, 192 patients had at least one cataract risk factor.
Conclusion
Sustained platelet increases and reduced bleeding symptoms were observed in EPAG-treated patients with cITP throughout the study. Concomitant ITP medications were reduced without requiring rescue medications. EPAG was well tolerated with exposures 6 years. Monitoring of HBLA and cataracts is appropriate, even with long-term treatment. Funding: Study (NCT00351468) is/remains sponsored by GlaxoSmithKline; EPAG is an asset of Novartis AG as of March 2, 2015.

Session topic: Platelet disorders 1
Keyword(s): Chronic ITP, Platelet count, Safety, Thrombopoietin (TPO)
{{ help_message }}
{{filter}}


