PHASE 1B RESULTS OF IDASANUTLIN + CYTARABINE (ARA-C) IN ACUTE MYELOID LEUKEMIA (AML) PATIENTS (PTS)
(Abstract release date: 05/19/16)
EHA Library. Pappayannidis C. 06/11/16; 135260; S504

Dr. Cristina Pappayannidis
Contributions
Contributions
Abstract
Abstract: S504
Type: Oral Presentation
Presentation during EHA21: On Saturday, June 11, 2016 from 16:45 - 17:00
Location: Hall A3
Background
Idasanutlin is a potent, oral MDM2 antagonist. A Phase 1b study was conducted to assess the safety and efficacy of idasanutlin + ara-C in relapsed/refractory (R/R) AML pts.
Aims
The primary endpoint was to identify the maximum tolerated dose and/or recommended dose and safety profile of idasanutlin + ara-C. Secondary endpoints included complete remission (CR) rate, complete remission with incomplete platelet (CRp) or hematologic recovery (CRi), morphologic leukemia free state (MLFS), composite complete remission (CRc, CR + CRp + CRi) rate, pharmacokinetics (PK), and exploratory analyses of predictive/prognostic biomarkers
Methods
In the dose escalation phase, R/R AML pts or those not considered candidates for standard induction therapies were treated with escalating doses of the initial idasanutlin formulation daily x 5 days (d) + ara-C 1 g/m2 x 6d. After identification of the recommended dose, an expansion cohort in R/R AML pts treated with ≤ 2 prior regimens was enrolled. A bridging arm was added to characterize the safety and PK of a spray dried powder (SDP) formulation of idasanutlin. CRs were confirmed ≥28d after initial assessment. TP53 mutation and flow cytometry of MDM2 expression in blasts were assessed as potentially predictive/prognostic by association with CRc.
Results
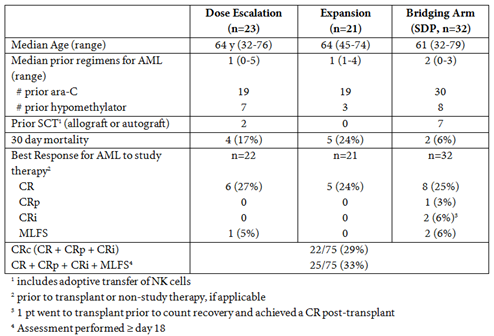
A total of 76 pts (1 pt was subsequently identified to have CMML and not AML) were treated with the combination therapy. Dose escalation patients (n=23) were treated with 400 mg qd (n=10), 400 mg bid (n=7), or 600 mg bid (n=6) of idasanutlin with ara-C. While not meeting protocol specified DLT criteria, diarrhea incidence and grade tracked with increasing dose and thus the recommended dose was 600 mg bid. Twenty-one pts were subsequently treated in an expansion cohort with 600 mg bid idasanutlin + ara-C. Thirty-two pts were enrolled in the bridging arm with either 300 mg bid (n=19) or 400 mg bid (n=13) of SDP idasanutlin + ara-C.Demographics and best responses are noted in the Table. The CR proportion was 25% (19/75 pts); the CRc proportion was 29% (22/75 pts), and the CR + CRp + CRi + MLFS proportion was 33% (25/75). Patients with CRc were followed until relapse or until 1 year (yr) from start of therapy; median duration of response is ~6.4 months (range 1.1 to 11.9 mos). Five pts remain in CR and continue in the 1 yr follow up period; 4 pts were in CR at the final visit ~1 yr from start of treatment. PK exhibited Cmax at ~6h, t1/2 of 1d, and dose proportionality. The SDP formulation doubled relative bioavailability.MDM2% positivity in CD45dim AML blasts by flow cytometry suggests that higher levels of MDM2 expression are associated with response (p=0.00049). TP53 mutation status was not associated but did trend with response (p=0.08) as predictive/prognostic association derives from negative predictive value of the small proportion of mutant patients. By contrast, MDM2 protein expression by flow cytometry displayed pronounced association with CRc when analyses were restricted to TP53 WT-only patients (p=0.0021).
Conclusion
Treatment with idasanutlin + ara-C resulted in durable CRs. Five of the 22 pts who achieved a CRc (23%) proceeded to transplant following therapy, demonstrating that this combination is a promising therapeutic option in R/R AML. Biomarker data suggests that identifying pts in which TP53 activity may be therapeutically enhanced may provide for improved outcomes. A Phase 3 trial is open and accruing.
Session topic: New Compounds in AML Treatment
Keyword(s): Acute myeloid leukemia, AML
Type: Oral Presentation
Presentation during EHA21: On Saturday, June 11, 2016 from 16:45 - 17:00
Location: Hall A3
Background
Idasanutlin is a potent, oral MDM2 antagonist. A Phase 1b study was conducted to assess the safety and efficacy of idasanutlin + ara-C in relapsed/refractory (R/R) AML pts.
Aims
The primary endpoint was to identify the maximum tolerated dose and/or recommended dose and safety profile of idasanutlin + ara-C. Secondary endpoints included complete remission (CR) rate, complete remission with incomplete platelet (CRp) or hematologic recovery (CRi), morphologic leukemia free state (MLFS), composite complete remission (CRc, CR + CRp + CRi) rate, pharmacokinetics (PK), and exploratory analyses of predictive/prognostic biomarkers
Methods
In the dose escalation phase, R/R AML pts or those not considered candidates for standard induction therapies were treated with escalating doses of the initial idasanutlin formulation daily x 5 days (d) + ara-C 1 g/m2 x 6d. After identification of the recommended dose, an expansion cohort in R/R AML pts treated with ≤ 2 prior regimens was enrolled. A bridging arm was added to characterize the safety and PK of a spray dried powder (SDP) formulation of idasanutlin. CRs were confirmed ≥28d after initial assessment. TP53 mutation and flow cytometry of MDM2 expression in blasts were assessed as potentially predictive/prognostic by association with CRc.
Results
A total of 76 pts (1 pt was subsequently identified to have CMML and not AML) were treated with the combination therapy. Dose escalation patients (n=23) were treated with 400 mg qd (n=10), 400 mg bid (n=7), or 600 mg bid (n=6) of idasanutlin with ara-C. While not meeting protocol specified DLT criteria, diarrhea incidence and grade tracked with increasing dose and thus the recommended dose was 600 mg bid. Twenty-one pts were subsequently treated in an expansion cohort with 600 mg bid idasanutlin + ara-C. Thirty-two pts were enrolled in the bridging arm with either 300 mg bid (n=19) or 400 mg bid (n=13) of SDP idasanutlin + ara-C.Demographics and best responses are noted in the Table. The CR proportion was 25% (19/75 pts); the CRc proportion was 29% (22/75 pts), and the CR + CRp + CRi + MLFS proportion was 33% (25/75). Patients with CRc were followed until relapse or until 1 year (yr) from start of therapy; median duration of response is ~6.4 months (range 1.1 to 11.9 mos). Five pts remain in CR and continue in the 1 yr follow up period; 4 pts were in CR at the final visit ~1 yr from start of treatment. PK exhibited Cmax at ~6h, t1/2 of 1d, and dose proportionality. The SDP formulation doubled relative bioavailability.MDM2% positivity in CD45dim AML blasts by flow cytometry suggests that higher levels of MDM2 expression are associated with response (p=0.00049). TP53 mutation status was not associated but did trend with response (p=0.08) as predictive/prognostic association derives from negative predictive value of the small proportion of mutant patients. By contrast, MDM2 protein expression by flow cytometry displayed pronounced association with CRc when analyses were restricted to TP53 WT-only patients (p=0.0021).
Conclusion
Treatment with idasanutlin + ara-C resulted in durable CRs. Five of the 22 pts who achieved a CRc (23%) proceeded to transplant following therapy, demonstrating that this combination is a promising therapeutic option in R/R AML. Biomarker data suggests that identifying pts in which TP53 activity may be therapeutically enhanced may provide for improved outcomes. A Phase 3 trial is open and accruing.

Session topic: New Compounds in AML Treatment
Keyword(s): Acute myeloid leukemia, AML
Abstract: S504
Type: Oral Presentation
Presentation during EHA21: On Saturday, June 11, 2016 from 16:45 - 17:00
Location: Hall A3
Background
Idasanutlin is a potent, oral MDM2 antagonist. A Phase 1b study was conducted to assess the safety and efficacy of idasanutlin + ara-C in relapsed/refractory (R/R) AML pts.
Aims
The primary endpoint was to identify the maximum tolerated dose and/or recommended dose and safety profile of idasanutlin + ara-C. Secondary endpoints included complete remission (CR) rate, complete remission with incomplete platelet (CRp) or hematologic recovery (CRi), morphologic leukemia free state (MLFS), composite complete remission (CRc, CR + CRp + CRi) rate, pharmacokinetics (PK), and exploratory analyses of predictive/prognostic biomarkers
Methods
In the dose escalation phase, R/R AML pts or those not considered candidates for standard induction therapies were treated with escalating doses of the initial idasanutlin formulation daily x 5 days (d) + ara-C 1 g/m2 x 6d. After identification of the recommended dose, an expansion cohort in R/R AML pts treated with ≤ 2 prior regimens was enrolled. A bridging arm was added to characterize the safety and PK of a spray dried powder (SDP) formulation of idasanutlin. CRs were confirmed ≥28d after initial assessment. TP53 mutation and flow cytometry of MDM2 expression in blasts were assessed as potentially predictive/prognostic by association with CRc.
Results
A total of 76 pts (1 pt was subsequently identified to have CMML and not AML) were treated with the combination therapy. Dose escalation patients (n=23) were treated with 400 mg qd (n=10), 400 mg bid (n=7), or 600 mg bid (n=6) of idasanutlin with ara-C. While not meeting protocol specified DLT criteria, diarrhea incidence and grade tracked with increasing dose and thus the recommended dose was 600 mg bid. Twenty-one pts were subsequently treated in an expansion cohort with 600 mg bid idasanutlin + ara-C. Thirty-two pts were enrolled in the bridging arm with either 300 mg bid (n=19) or 400 mg bid (n=13) of SDP idasanutlin + ara-C.Demographics and best responses are noted in the Table. The CR proportion was 25% (19/75 pts); the CRc proportion was 29% (22/75 pts), and the CR + CRp + CRi + MLFS proportion was 33% (25/75). Patients with CRc were followed until relapse or until 1 year (yr) from start of therapy; median duration of response is ~6.4 months (range 1.1 to 11.9 mos). Five pts remain in CR and continue in the 1 yr follow up period; 4 pts were in CR at the final visit ~1 yr from start of treatment. PK exhibited Cmax at ~6h, t1/2 of 1d, and dose proportionality. The SDP formulation doubled relative bioavailability.MDM2% positivity in CD45dim AML blasts by flow cytometry suggests that higher levels of MDM2 expression are associated with response (p=0.00049). TP53 mutation status was not associated but did trend with response (p=0.08) as predictive/prognostic association derives from negative predictive value of the small proportion of mutant patients. By contrast, MDM2 protein expression by flow cytometry displayed pronounced association with CRc when analyses were restricted to TP53 WT-only patients (p=0.0021).
Conclusion
Treatment with idasanutlin + ara-C resulted in durable CRs. Five of the 22 pts who achieved a CRc (23%) proceeded to transplant following therapy, demonstrating that this combination is a promising therapeutic option in R/R AML. Biomarker data suggests that identifying pts in which TP53 activity may be therapeutically enhanced may provide for improved outcomes. A Phase 3 trial is open and accruing.

Session topic: New Compounds in AML Treatment
Keyword(s): Acute myeloid leukemia, AML
Type: Oral Presentation
Presentation during EHA21: On Saturday, June 11, 2016 from 16:45 - 17:00
Location: Hall A3
Background
Idasanutlin is a potent, oral MDM2 antagonist. A Phase 1b study was conducted to assess the safety and efficacy of idasanutlin + ara-C in relapsed/refractory (R/R) AML pts.
Aims
The primary endpoint was to identify the maximum tolerated dose and/or recommended dose and safety profile of idasanutlin + ara-C. Secondary endpoints included complete remission (CR) rate, complete remission with incomplete platelet (CRp) or hematologic recovery (CRi), morphologic leukemia free state (MLFS), composite complete remission (CRc, CR + CRp + CRi) rate, pharmacokinetics (PK), and exploratory analyses of predictive/prognostic biomarkers
Methods
In the dose escalation phase, R/R AML pts or those not considered candidates for standard induction therapies were treated with escalating doses of the initial idasanutlin formulation daily x 5 days (d) + ara-C 1 g/m2 x 6d. After identification of the recommended dose, an expansion cohort in R/R AML pts treated with ≤ 2 prior regimens was enrolled. A bridging arm was added to characterize the safety and PK of a spray dried powder (SDP) formulation of idasanutlin. CRs were confirmed ≥28d after initial assessment. TP53 mutation and flow cytometry of MDM2 expression in blasts were assessed as potentially predictive/prognostic by association with CRc.
Results
A total of 76 pts (1 pt was subsequently identified to have CMML and not AML) were treated with the combination therapy. Dose escalation patients (n=23) were treated with 400 mg qd (n=10), 400 mg bid (n=7), or 600 mg bid (n=6) of idasanutlin with ara-C. While not meeting protocol specified DLT criteria, diarrhea incidence and grade tracked with increasing dose and thus the recommended dose was 600 mg bid. Twenty-one pts were subsequently treated in an expansion cohort with 600 mg bid idasanutlin + ara-C. Thirty-two pts were enrolled in the bridging arm with either 300 mg bid (n=19) or 400 mg bid (n=13) of SDP idasanutlin + ara-C.Demographics and best responses are noted in the Table. The CR proportion was 25% (19/75 pts); the CRc proportion was 29% (22/75 pts), and the CR + CRp + CRi + MLFS proportion was 33% (25/75). Patients with CRc were followed until relapse or until 1 year (yr) from start of therapy; median duration of response is ~6.4 months (range 1.1 to 11.9 mos). Five pts remain in CR and continue in the 1 yr follow up period; 4 pts were in CR at the final visit ~1 yr from start of treatment. PK exhibited Cmax at ~6h, t1/2 of 1d, and dose proportionality. The SDP formulation doubled relative bioavailability.MDM2% positivity in CD45dim AML blasts by flow cytometry suggests that higher levels of MDM2 expression are associated with response (p=0.00049). TP53 mutation status was not associated but did trend with response (p=0.08) as predictive/prognostic association derives from negative predictive value of the small proportion of mutant patients. By contrast, MDM2 protein expression by flow cytometry displayed pronounced association with CRc when analyses were restricted to TP53 WT-only patients (p=0.0021).
Conclusion
Treatment with idasanutlin + ara-C resulted in durable CRs. Five of the 22 pts who achieved a CRc (23%) proceeded to transplant following therapy, demonstrating that this combination is a promising therapeutic option in R/R AML. Biomarker data suggests that identifying pts in which TP53 activity may be therapeutically enhanced may provide for improved outcomes. A Phase 3 trial is open and accruing.

Session topic: New Compounds in AML Treatment
Keyword(s): Acute myeloid leukemia, AML
{{ help_message }}
{{filter}}


