Department of hematology

Contributions
Type: Publication Only
Background
Bortezomib is a proteasome inhibitor which has proven potent activity against malignant plasma cells.Peripheral polyneuropathy (PNP)is one of the most frequent, non-hematologic adverse effects (AE) of Bortezomib often requiring dose modification or discontinuation.
Aims
Although the initial report by Moreau et al demonstrating the advantages and safety of subcutaneous use which led to the approval,this route of application is not being widely used in Turkey.Here we aimed to present our experience on the incidence of Bortezomib induced neuropathy (BiN)comparing two approved routes of administration.
Methods
After 2012 s.c.administration has become an accepted approach in our clinic for patients presenting with BiNin addition to dose reduction as recommended in the package.Weekly administration is the second step that follows if PNP does not improve.After 2013 s.c.has become the preferred approach for NDMM too.We retrospectively analyzed 129 consecutive patients diagnosed with Multiple Myeloma at Ankara University School of Medicine Department of Hematology between 2008-2013 and had completed at least three cycles of a Bortezomib containing protocol either at diagnosis or at relapse. Hospital records were screened to evaluate the incidence ofBiN, dose adjustments arising from AEs of Bortezomib and outcome measures.We compared the frequencies of AEs and treatment outcomes between patients who started treatment with s.c.versus i.v.administration of Bortezomib.We also analyzed outcomes following conversion from iv to sc too.Statistical analysis were performed using the SPSS for Windows,Version 16.0
Results
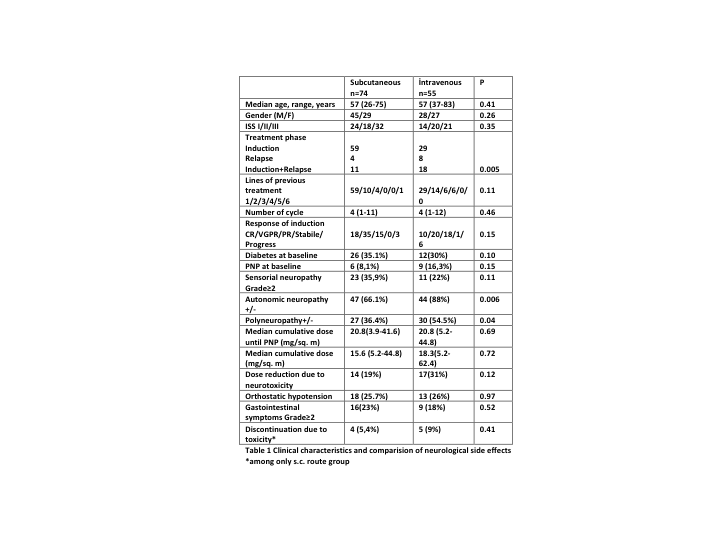
Baseline demographics,disease characteristics or number of previous lines of therapy were similar between patients receiving s.c.or i.v.treatment(Table-1).De novo subcutaneous Bortezomibwas given to 58 patients during induction;16 patients converted from i.v.tos.c route following diagnosis of BiN(n:9) or due to change in adminstration policy(n:7).s.c.groupconsisted of 74 patients.55 Patients completed Bortezomib treatment only i.v.The median cumulative dose of Bortezomib was 15.6 (range 5.2-44.8) and 18.3 (range 5.2-62.4) mg/sq.m. among sc and iv groups (p=0.72).Neurologic adverse effects are summarized in Table-1.The median cumulative Bortezomib dose to onset of any grade of PN was 20.8 (range 3.9-41.6) and 20.8 (range 5.2-44.8) mg/sq.m. to onset of PN with sc and iv group (p=0.69)respectively. Although statistically not significant dose reduction was required more often among patients in the iv group.Eight of the nine patients who converted to sc following onset of BiN had a improvement in symptoms with change in route and weekly administration.None of the patients experienced severe AEs related to s.c. administration or discontinued treatment. Rash at injection site was the most frequent AE which was lasting for 3 days maximum and easily tolerable.The progression-free survival (PFS) and OS between arms were also similar.
Summary
These results confirmed the safety and equal efficacy of sc Bortezomib compared to iv administration.Based on our results we recommend s.c. administration to avoid dose reduction requirement and discontinuation arising from neurotoxicity.
Keyword(s): Bortezomib, Toxicity
Session topic: Publication Only
Type: Publication Only
Background
Bortezomib is a proteasome inhibitor which has proven potent activity against malignant plasma cells.Peripheral polyneuropathy (PNP)is one of the most frequent, non-hematologic adverse effects (AE) of Bortezomib often requiring dose modification or discontinuation.
Aims
Although the initial report by Moreau et al demonstrating the advantages and safety of subcutaneous use which led to the approval,this route of application is not being widely used in Turkey.Here we aimed to present our experience on the incidence of Bortezomib induced neuropathy (BiN)comparing two approved routes of administration.
Methods
After 2012 s.c.administration has become an accepted approach in our clinic for patients presenting with BiNin addition to dose reduction as recommended in the package.Weekly administration is the second step that follows if PNP does not improve.After 2013 s.c.has become the preferred approach for NDMM too.We retrospectively analyzed 129 consecutive patients diagnosed with Multiple Myeloma at Ankara University School of Medicine Department of Hematology between 2008-2013 and had completed at least three cycles of a Bortezomib containing protocol either at diagnosis or at relapse. Hospital records were screened to evaluate the incidence ofBiN, dose adjustments arising from AEs of Bortezomib and outcome measures.We compared the frequencies of AEs and treatment outcomes between patients who started treatment with s.c.versus i.v.administration of Bortezomib.We also analyzed outcomes following conversion from iv to sc too.Statistical analysis were performed using the SPSS for Windows,Version 16.0
Results
Baseline demographics,disease characteristics or number of previous lines of therapy were similar between patients receiving s.c.or i.v.treatment(Table-1).De novo subcutaneous Bortezomibwas given to 58 patients during induction;16 patients converted from i.v.tos.c route following diagnosis of BiN(n:9) or due to change in adminstration policy(n:7).s.c.groupconsisted of 74 patients.55 Patients completed Bortezomib treatment only i.v.The median cumulative dose of Bortezomib was 15.6 (range 5.2-44.8) and 18.3 (range 5.2-62.4) mg/sq.m. among sc and iv groups (p=0.72).Neurologic adverse effects are summarized in Table-1.The median cumulative Bortezomib dose to onset of any grade of PN was 20.8 (range 3.9-41.6) and 20.8 (range 5.2-44.8) mg/sq.m. to onset of PN with sc and iv group (p=0.69)respectively. Although statistically not significant dose reduction was required more often among patients in the iv group.Eight of the nine patients who converted to sc following onset of BiN had a improvement in symptoms with change in route and weekly administration.None of the patients experienced severe AEs related to s.c. administration or discontinued treatment. Rash at injection site was the most frequent AE which was lasting for 3 days maximum and easily tolerable.The progression-free survival (PFS) and OS between arms were also similar.
Summary
These results confirmed the safety and equal efficacy of sc Bortezomib compared to iv administration.Based on our results we recommend s.c. administration to avoid dose reduction requirement and discontinuation arising from neurotoxicity.
Keyword(s): Bortezomib, Toxicity

Session topic: Publication Only


