
Contributions
Type: Publication Only
Background
The 2012 FDA approval of subcutaneous (SC) bortezomib (BTZ) was based on non-inferior efficacy and reduced toxicity vs intravenous (IV) administration of BTZ. While differences in BTZ toxicity are attributed to route, dose, schedule, and duration, little is known about BTZ use in recent real-world practice in the US.
Aims
This study aimed to examine the real-world use of bortezomib in relapsed/refractory multiple myeloma patients in the US.
Methods
Patients (pts) with relapsed/refractory (R/R) multiple myeloma (MM) were selected from a longitudinal, nationally-representative electronic medical record (EMR) database (Flatiron Health). MM diagnoses were confirmed by physician (MD) notes. Pts were required to have disease progression following 1st line of therapy (LOT), ≥ 1 visit after 2010, and ≥ 3 months follow-up post-progression. Pt-level data were integrated from structured and unstructured EMR sources: transplant status abstracted from MD notes and IV/SC administration data merged with oral data from MD notes. We evaluated BTZ usage among these pts with R/R MM.
Results
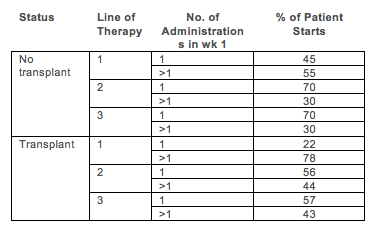
We identified 607 pts with R/R MM; among these pts, 542 (89%) received BTZ. There were 830 LOTs containing BTZ. Initiation with SC vs IV BTZ increased over time (2011: 13% SC; 2012: 64% SC; 2013: 76% SC; 2014: 71% SC). Among BTZ initiations after 2012, 34% of those starting IV BTZ later switched to SC (7% of SC BTZ initiations later switched to IV). Across all BTZ initiations, 47% started at a schedule of > once per week (1×/wk); the rate was higher in earlier LOTs and transplant recipients (Table 1). BTZ initiations starting at 1x/wk were associated with longer duration (4.9 vs. 4.0 months, p=0.02), fewer dose reductions (26% vs. 40%, p<0.01) and similar cumulative total dose (median of 20.8 vs. 22.3 mg/m2, p=0.24) than BTZ initiations starting at >1x/wk.
Summary
To our knowledge, this is the first real-world study evaluating evolving patterns of BTZ use in pts with R/R MM in the US. There was increased SC administration after 2012, longer duration of treatment among those starting at 1x/wk, and fewer dose reductions among those starting at 1x/wk; all which may be associated with toxicity management.
Session topic: Publication Only
Type: Publication Only
Background
The 2012 FDA approval of subcutaneous (SC) bortezomib (BTZ) was based on non-inferior efficacy and reduced toxicity vs intravenous (IV) administration of BTZ. While differences in BTZ toxicity are attributed to route, dose, schedule, and duration, little is known about BTZ use in recent real-world practice in the US.
Aims
This study aimed to examine the real-world use of bortezomib in relapsed/refractory multiple myeloma patients in the US.
Methods
Patients (pts) with relapsed/refractory (R/R) multiple myeloma (MM) were selected from a longitudinal, nationally-representative electronic medical record (EMR) database (Flatiron Health). MM diagnoses were confirmed by physician (MD) notes. Pts were required to have disease progression following 1st line of therapy (LOT), ≥ 1 visit after 2010, and ≥ 3 months follow-up post-progression. Pt-level data were integrated from structured and unstructured EMR sources: transplant status abstracted from MD notes and IV/SC administration data merged with oral data from MD notes. We evaluated BTZ usage among these pts with R/R MM.
Results
We identified 607 pts with R/R MM; among these pts, 542 (89%) received BTZ. There were 830 LOTs containing BTZ. Initiation with SC vs IV BTZ increased over time (2011: 13% SC; 2012: 64% SC; 2013: 76% SC; 2014: 71% SC). Among BTZ initiations after 2012, 34% of those starting IV BTZ later switched to SC (7% of SC BTZ initiations later switched to IV). Across all BTZ initiations, 47% started at a schedule of > once per week (1×/wk); the rate was higher in earlier LOTs and transplant recipients (Table 1). BTZ initiations starting at 1x/wk were associated with longer duration (4.9 vs. 4.0 months, p=0.02), fewer dose reductions (26% vs. 40%, p<0.01) and similar cumulative total dose (median of 20.8 vs. 22.3 mg/m2, p=0.24) than BTZ initiations starting at >1x/wk.
Summary
To our knowledge, this is the first real-world study evaluating evolving patterns of BTZ use in pts with R/R MM in the US. There was increased SC administration after 2012, longer duration of treatment among those starting at 1x/wk, and fewer dose reductions among those starting at 1x/wk; all which may be associated with toxicity management.

Session topic: Publication Only


