
Contributions
Abstract: S780
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:15 - 08:30
Location: Hall D
Background
Addition of a proteasome inhibitor to a doublet backbone therapy has been shown to improve efficacy in newly diagnosed multiple myeloma (NDMM) patients (San Miguel et al, N Engl J Med 2008, Durie et al, Lancet 2017). Data from two phase 1/2 studies indicate that the combination of ixazomib plus lenalidomide-dexamethasone (IRd) is feasible and active in patients with NDMM, with weekly and twice-weekly ixazomib dosing having been investigated (Kumar et al, Lancet Oncol 2014; Richardson et al, Blood 2013).
Aims
This phase 1/2 study (NCT01383928) evaluated twice-weekly ixazomib plus Rd as induction therapy, followed by maintenance therapy with single-agent ixazomib. We report long-term efficacy and safety data in patients who did not withdraw from the study in order to receive SCT.
Methods
Patients with NDMM (SCT-eligible or SCT-ineligible) received twice-weekly oral ixazomib (3.0 or 3.7 mg on days 1, 4, 8, and 11) plus lenalidomide (25 mg on days 1–14) and dexamethasone (20 mg [10 mg in cycles 9–16] on days 1, 2, 4, 5, 8, 9, 11, and 12) for up to sixteen 21-day cycles, followed by maintenance therapy with single-agent twice-weekly ixazomib. Patients received therapy until disease progression or toxicity. Those who proceeded to SCT did not receive further ixazomib therapy. Response/progression was assessed per IMWG criteria after cycles 1, 2, 3, 4, and then every 2 cycles during induction and maintenance.
Results
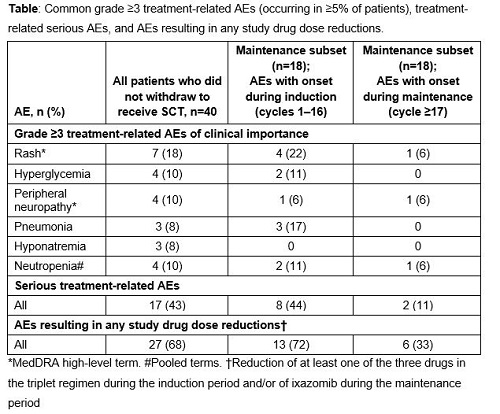
Of the 64 enrolled patients, 40 continued on study treatment without early withdrawal for SCT; long-term follow-up of these 40 patients is reported here. The median age of patients was 66 years (range 34–82), and 45%/38%/18% of patients had ISS disease stage I/II/III. At a median follow-up of 47.0 months, the overall response rate (ORR; ≥partial response [PR]) in the response-evaluable population was 95%, the complete plus very good partial response (CR+VGPR) rate was 68%, and the CR rate was 32%. Median time to first response was approximately 1 cycle (0.72 months). Median time to a best response of ≥CR was 4.2 months. Patients received a median (range) of 14 (1–75) treatment cycles. Median progression-free survival (PFS) for patients not proceeding to SCT was 24.9 months. Median overall survival (OS) was not estimable; the 2-year Kaplan-Meier estimate for OS was 92%. A total of 78% of patients had grade ≥3 treatment-related adverse events (AEs); the most common treatment-related grade ≥3 AEs and serious AEs are shown in the Table.
Conclusion
In patients with NDMM, twice-weekly ixazomib plus Rd resulted in exceptional response rates in patients who did not receive a SCT and who received maintenance therapy. The responses were deep and durable, with long PFS and a high 2-year OS estimate. The majority of AEs had an onset during induction, and the incidence of AEs during maintenance was infrequent.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Survival, Proteasome inhibitor, Oral, Myeloma
Abstract: S780
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:15 - 08:30
Location: Hall D
Background
Addition of a proteasome inhibitor to a doublet backbone therapy has been shown to improve efficacy in newly diagnosed multiple myeloma (NDMM) patients (San Miguel et al, N Engl J Med 2008, Durie et al, Lancet 2017). Data from two phase 1/2 studies indicate that the combination of ixazomib plus lenalidomide-dexamethasone (IRd) is feasible and active in patients with NDMM, with weekly and twice-weekly ixazomib dosing having been investigated (Kumar et al, Lancet Oncol 2014; Richardson et al, Blood 2013).
Aims
This phase 1/2 study (NCT01383928) evaluated twice-weekly ixazomib plus Rd as induction therapy, followed by maintenance therapy with single-agent ixazomib. We report long-term efficacy and safety data in patients who did not withdraw from the study in order to receive SCT.
Methods
Patients with NDMM (SCT-eligible or SCT-ineligible) received twice-weekly oral ixazomib (3.0 or 3.7 mg on days 1, 4, 8, and 11) plus lenalidomide (25 mg on days 1–14) and dexamethasone (20 mg [10 mg in cycles 9–16] on days 1, 2, 4, 5, 8, 9, 11, and 12) for up to sixteen 21-day cycles, followed by maintenance therapy with single-agent twice-weekly ixazomib. Patients received therapy until disease progression or toxicity. Those who proceeded to SCT did not receive further ixazomib therapy. Response/progression was assessed per IMWG criteria after cycles 1, 2, 3, 4, and then every 2 cycles during induction and maintenance.
Results
Of the 64 enrolled patients, 40 continued on study treatment without early withdrawal for SCT; long-term follow-up of these 40 patients is reported here. The median age of patients was 66 years (range 34–82), and 45%/38%/18% of patients had ISS disease stage I/II/III. At a median follow-up of 47.0 months, the overall response rate (ORR; ≥partial response [PR]) in the response-evaluable population was 95%, the complete plus very good partial response (CR+VGPR) rate was 68%, and the CR rate was 32%. Median time to first response was approximately 1 cycle (0.72 months). Median time to a best response of ≥CR was 4.2 months. Patients received a median (range) of 14 (1–75) treatment cycles. Median progression-free survival (PFS) for patients not proceeding to SCT was 24.9 months. Median overall survival (OS) was not estimable; the 2-year Kaplan-Meier estimate for OS was 92%. A total of 78% of patients had grade ≥3 treatment-related adverse events (AEs); the most common treatment-related grade ≥3 AEs and serious AEs are shown in the Table.

Conclusion
In patients with NDMM, twice-weekly ixazomib plus Rd resulted in exceptional response rates in patients who did not receive a SCT and who received maintenance therapy. The responses were deep and durable, with long PFS and a high 2-year OS estimate. The majority of AEs had an onset during induction, and the incidence of AEs during maintenance was infrequent.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Survival, Proteasome inhibitor, Oral, Myeloma


