A PHASE 2, OPEN-LABEL, MULTICENTER STUDY OF ELOTUZUMAB MONOTHERAPY IN PATIENTS WITH HIGH-RISK SMOLDERING MULTIPLE MYELOMA
(Abstract release date: 05/19/16)
EHA Library. Jagannath S. 06/12/16; 135309; S815

Prof. Dr. Sundar Jagannath
Contributions
Contributions
Abstract
Abstract: S815
Type: Oral Presentation
Presentation during EHA21: On Sunday, June 12, 2016 from 08:30 - 08:45
Location: Hall C14
Background
Smoldering multiple myeloma (SMM) is an asymptomatic precursor of active multiple myeloma (MM), with no approved therapies. Patients (pts) with MM have compromised natural killer (NK) cell function; during early disease, pts may have better immune activity. Elotuzumab is an IgG1 immunostimulatory antibody targeted against SLAMF7, a glycoprotein expressed by myeloma and NK cells.1 Progression from SMM to MM, and association between NK cell status and tumor burden, is incompletely understood. SMM provides a disease setting to assess elotuzumab monotherapy in less immune compromised pts.
Aims
To explore the association between baseline (BL) CD56dim NK cells in bone marrow and maximal change in serum M protein (measure of tumor burden) in pts with high-risk SMM treated with elotuzumab. Efficacy and safety of elotuzumab monotherapy were also assessed.
Methods
Pts with high-risk SMM participated in this Ph 2, open-label study (NCT01441973). High risk was defined as serum M protein ≥3 g/dL and bone marrow plasma cells (BMPCs) ≥10% (criteria 1); serum M protein 1–3 g/dL, BMPCs ≥10%, and an abnormal free light chain (FLC) ratio (<0.125 or >8.0) (criteria 2); or urine M protein >200 mg/24 h, BMPC ≥10%, and an abnormal FLC (≤0.125 or ≥8.0; criteria 3).2 Two high-risk cohorts, enrolled serially, were examined to explore different dosing schedules: pts in cohort 1 (C1) received elotuzumab 20 mg/kg IV in 28-day cycles (cycle 1: Days 1 and 8; cycle 2+: once monthly); in cohort 2 (C2), pts received elotuzumab 10 mg/kg IV in 28-day cycles (cycles 1 and 2: wkly; cycle 3+: twice monthly). Tumor response was assessed every 4 wks. Pts received elotuzumab until disease progression per modified International Myeloma Working Group (IMWG) criteria (including progressive disease or progression to symptomatic active myeloma). Primary endpoint was association between BL percent of bone marrow CD56dim NK cells (CD45+/CD3-/CD56dim/CD16+) and maximal change in serum M protein. Secondary endpoints were progression-free survival (PFS) and overall response rate (ORR). Safety was an exploratory endpoint. Informed written consent was obtained for all pts.
Results
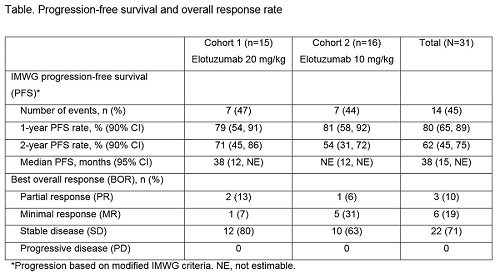
31 pts were treated (C1, n=15; C2, n=16). Across both cohorts, 32% of pts met criteria 1 for high-risk SMM, 61% met criteria 2, 13% met criteria 3. At data cut-off (January 26, 2016), 27% and 38% of pts from C1 and C2, respectively, were still on treatment. Main reason for discontinuation was disease progression (C1, 47%; C2, 31%). A clear association between BL percent CD56dim cells and maximal change in serum M protein (p=0.779) and between BL CD56dim cells and response (≥minimal response; p=0.988) was not established. PFS and ORR are reported (Table). AEs were reported in all pts in both cohorts. No grade 5 AEs were reported. Most common AEs of any grade were upper respiratory tract infection (C1, 53%; C2, 63%) and fatigue (C1, 47%; C2, 38%). Infusion reactions were reported in 13% of pts; all grade 1–2. SAEs were reported in 53% of pts in C1 and 44% in C2.
Conclusion
An association between BL percent CD56dim NK cells in bone marrow and changes in serum M protein with elotuzumab treatment was not established. Elotuzumab monotherapy may delay SMM progression to MM, as most pts achieved a best overall response of SD or MR, with ≥MR in 29% of pts, including PR in 10%, and favorable PFS. Treatment was generally well tolerated; the AE profile was consistent with prior elotuzumab experience. Funding: BMS. Medical writing: S Addison, Caudex, funded by BMS. 1. Hsi E et al. Clin Cancer Res 2008;14:2775–84
2. Kyle R et. al. Leukemia 2010;24:1121–27
Session topic: Experimental approaches for plasma cell disorders
Keyword(s): Monoclonal antibody, Multiple myeloma, Smoldering
Type: Oral Presentation
Presentation during EHA21: On Sunday, June 12, 2016 from 08:30 - 08:45
Location: Hall C14
Background
Smoldering multiple myeloma (SMM) is an asymptomatic precursor of active multiple myeloma (MM), with no approved therapies. Patients (pts) with MM have compromised natural killer (NK) cell function; during early disease, pts may have better immune activity. Elotuzumab is an IgG1 immunostimulatory antibody targeted against SLAMF7, a glycoprotein expressed by myeloma and NK cells.1 Progression from SMM to MM, and association between NK cell status and tumor burden, is incompletely understood. SMM provides a disease setting to assess elotuzumab monotherapy in less immune compromised pts.
Aims
To explore the association between baseline (BL) CD56dim NK cells in bone marrow and maximal change in serum M protein (measure of tumor burden) in pts with high-risk SMM treated with elotuzumab. Efficacy and safety of elotuzumab monotherapy were also assessed.
Methods
Pts with high-risk SMM participated in this Ph 2, open-label study (NCT01441973). High risk was defined as serum M protein ≥3 g/dL and bone marrow plasma cells (BMPCs) ≥10% (criteria 1); serum M protein 1–3 g/dL, BMPCs ≥10%, and an abnormal free light chain (FLC) ratio (<0.125 or >8.0) (criteria 2); or urine M protein >200 mg/24 h, BMPC ≥10%, and an abnormal FLC (≤0.125 or ≥8.0; criteria 3).2 Two high-risk cohorts, enrolled serially, were examined to explore different dosing schedules: pts in cohort 1 (C1) received elotuzumab 20 mg/kg IV in 28-day cycles (cycle 1: Days 1 and 8; cycle 2+: once monthly); in cohort 2 (C2), pts received elotuzumab 10 mg/kg IV in 28-day cycles (cycles 1 and 2: wkly; cycle 3+: twice monthly). Tumor response was assessed every 4 wks. Pts received elotuzumab until disease progression per modified International Myeloma Working Group (IMWG) criteria (including progressive disease or progression to symptomatic active myeloma). Primary endpoint was association between BL percent of bone marrow CD56dim NK cells (CD45+/CD3-/CD56dim/CD16+) and maximal change in serum M protein. Secondary endpoints were progression-free survival (PFS) and overall response rate (ORR). Safety was an exploratory endpoint. Informed written consent was obtained for all pts.
Results
31 pts were treated (C1, n=15; C2, n=16). Across both cohorts, 32% of pts met criteria 1 for high-risk SMM, 61% met criteria 2, 13% met criteria 3. At data cut-off (January 26, 2016), 27% and 38% of pts from C1 and C2, respectively, were still on treatment. Main reason for discontinuation was disease progression (C1, 47%; C2, 31%). A clear association between BL percent CD56dim cells and maximal change in serum M protein (p=0.779) and between BL CD56dim cells and response (≥minimal response; p=0.988) was not established. PFS and ORR are reported (Table). AEs were reported in all pts in both cohorts. No grade 5 AEs were reported. Most common AEs of any grade were upper respiratory tract infection (C1, 53%; C2, 63%) and fatigue (C1, 47%; C2, 38%). Infusion reactions were reported in 13% of pts; all grade 1–2. SAEs were reported in 53% of pts in C1 and 44% in C2.
Conclusion
An association between BL percent CD56dim NK cells in bone marrow and changes in serum M protein with elotuzumab treatment was not established. Elotuzumab monotherapy may delay SMM progression to MM, as most pts achieved a best overall response of SD or MR, with ≥MR in 29% of pts, including PR in 10%, and favorable PFS. Treatment was generally well tolerated; the AE profile was consistent with prior elotuzumab experience. Funding: BMS. Medical writing: S Addison, Caudex, funded by BMS. 1. Hsi E et al. Clin Cancer Res 2008;14:2775–84
2. Kyle R et. al. Leukemia 2010;24:1121–27

Session topic: Experimental approaches for plasma cell disorders
Keyword(s): Monoclonal antibody, Multiple myeloma, Smoldering
Abstract: S815
Type: Oral Presentation
Presentation during EHA21: On Sunday, June 12, 2016 from 08:30 - 08:45
Location: Hall C14
Background
Smoldering multiple myeloma (SMM) is an asymptomatic precursor of active multiple myeloma (MM), with no approved therapies. Patients (pts) with MM have compromised natural killer (NK) cell function; during early disease, pts may have better immune activity. Elotuzumab is an IgG1 immunostimulatory antibody targeted against SLAMF7, a glycoprotein expressed by myeloma and NK cells.1 Progression from SMM to MM, and association between NK cell status and tumor burden, is incompletely understood. SMM provides a disease setting to assess elotuzumab monotherapy in less immune compromised pts.
Aims
To explore the association between baseline (BL) CD56dim NK cells in bone marrow and maximal change in serum M protein (measure of tumor burden) in pts with high-risk SMM treated with elotuzumab. Efficacy and safety of elotuzumab monotherapy were also assessed.
Methods
Pts with high-risk SMM participated in this Ph 2, open-label study (NCT01441973). High risk was defined as serum M protein ≥3 g/dL and bone marrow plasma cells (BMPCs) ≥10% (criteria 1); serum M protein 1–3 g/dL, BMPCs ≥10%, and an abnormal free light chain (FLC) ratio (<0.125 or >8.0) (criteria 2); or urine M protein >200 mg/24 h, BMPC ≥10%, and an abnormal FLC (≤0.125 or ≥8.0; criteria 3).2 Two high-risk cohorts, enrolled serially, were examined to explore different dosing schedules: pts in cohort 1 (C1) received elotuzumab 20 mg/kg IV in 28-day cycles (cycle 1: Days 1 and 8; cycle 2+: once monthly); in cohort 2 (C2), pts received elotuzumab 10 mg/kg IV in 28-day cycles (cycles 1 and 2: wkly; cycle 3+: twice monthly). Tumor response was assessed every 4 wks. Pts received elotuzumab until disease progression per modified International Myeloma Working Group (IMWG) criteria (including progressive disease or progression to symptomatic active myeloma). Primary endpoint was association between BL percent of bone marrow CD56dim NK cells (CD45+/CD3-/CD56dim/CD16+) and maximal change in serum M protein. Secondary endpoints were progression-free survival (PFS) and overall response rate (ORR). Safety was an exploratory endpoint. Informed written consent was obtained for all pts.
Results
31 pts were treated (C1, n=15; C2, n=16). Across both cohorts, 32% of pts met criteria 1 for high-risk SMM, 61% met criteria 2, 13% met criteria 3. At data cut-off (January 26, 2016), 27% and 38% of pts from C1 and C2, respectively, were still on treatment. Main reason for discontinuation was disease progression (C1, 47%; C2, 31%). A clear association between BL percent CD56dim cells and maximal change in serum M protein (p=0.779) and between BL CD56dim cells and response (≥minimal response; p=0.988) was not established. PFS and ORR are reported (Table). AEs were reported in all pts in both cohorts. No grade 5 AEs were reported. Most common AEs of any grade were upper respiratory tract infection (C1, 53%; C2, 63%) and fatigue (C1, 47%; C2, 38%). Infusion reactions were reported in 13% of pts; all grade 1–2. SAEs were reported in 53% of pts in C1 and 44% in C2.
Conclusion
An association between BL percent CD56dim NK cells in bone marrow and changes in serum M protein with elotuzumab treatment was not established. Elotuzumab monotherapy may delay SMM progression to MM, as most pts achieved a best overall response of SD or MR, with ≥MR in 29% of pts, including PR in 10%, and favorable PFS. Treatment was generally well tolerated; the AE profile was consistent with prior elotuzumab experience. Funding: BMS. Medical writing: S Addison, Caudex, funded by BMS. 1. Hsi E et al. Clin Cancer Res 2008;14:2775–84
2. Kyle R et. al. Leukemia 2010;24:1121–27

Session topic: Experimental approaches for plasma cell disorders
Keyword(s): Monoclonal antibody, Multiple myeloma, Smoldering
Type: Oral Presentation
Presentation during EHA21: On Sunday, June 12, 2016 from 08:30 - 08:45
Location: Hall C14
Background
Smoldering multiple myeloma (SMM) is an asymptomatic precursor of active multiple myeloma (MM), with no approved therapies. Patients (pts) with MM have compromised natural killer (NK) cell function; during early disease, pts may have better immune activity. Elotuzumab is an IgG1 immunostimulatory antibody targeted against SLAMF7, a glycoprotein expressed by myeloma and NK cells.1 Progression from SMM to MM, and association between NK cell status and tumor burden, is incompletely understood. SMM provides a disease setting to assess elotuzumab monotherapy in less immune compromised pts.
Aims
To explore the association between baseline (BL) CD56dim NK cells in bone marrow and maximal change in serum M protein (measure of tumor burden) in pts with high-risk SMM treated with elotuzumab. Efficacy and safety of elotuzumab monotherapy were also assessed.
Methods
Pts with high-risk SMM participated in this Ph 2, open-label study (NCT01441973). High risk was defined as serum M protein ≥3 g/dL and bone marrow plasma cells (BMPCs) ≥10% (criteria 1); serum M protein 1–3 g/dL, BMPCs ≥10%, and an abnormal free light chain (FLC) ratio (<0.125 or >8.0) (criteria 2); or urine M protein >200 mg/24 h, BMPC ≥10%, and an abnormal FLC (≤0.125 or ≥8.0; criteria 3).2 Two high-risk cohorts, enrolled serially, were examined to explore different dosing schedules: pts in cohort 1 (C1) received elotuzumab 20 mg/kg IV in 28-day cycles (cycle 1: Days 1 and 8; cycle 2+: once monthly); in cohort 2 (C2), pts received elotuzumab 10 mg/kg IV in 28-day cycles (cycles 1 and 2: wkly; cycle 3+: twice monthly). Tumor response was assessed every 4 wks. Pts received elotuzumab until disease progression per modified International Myeloma Working Group (IMWG) criteria (including progressive disease or progression to symptomatic active myeloma). Primary endpoint was association between BL percent of bone marrow CD56dim NK cells (CD45+/CD3-/CD56dim/CD16+) and maximal change in serum M protein. Secondary endpoints were progression-free survival (PFS) and overall response rate (ORR). Safety was an exploratory endpoint. Informed written consent was obtained for all pts.
Results
31 pts were treated (C1, n=15; C2, n=16). Across both cohorts, 32% of pts met criteria 1 for high-risk SMM, 61% met criteria 2, 13% met criteria 3. At data cut-off (January 26, 2016), 27% and 38% of pts from C1 and C2, respectively, were still on treatment. Main reason for discontinuation was disease progression (C1, 47%; C2, 31%). A clear association between BL percent CD56dim cells and maximal change in serum M protein (p=0.779) and between BL CD56dim cells and response (≥minimal response; p=0.988) was not established. PFS and ORR are reported (Table). AEs were reported in all pts in both cohorts. No grade 5 AEs were reported. Most common AEs of any grade were upper respiratory tract infection (C1, 53%; C2, 63%) and fatigue (C1, 47%; C2, 38%). Infusion reactions were reported in 13% of pts; all grade 1–2. SAEs were reported in 53% of pts in C1 and 44% in C2.
Conclusion
An association between BL percent CD56dim NK cells in bone marrow and changes in serum M protein with elotuzumab treatment was not established. Elotuzumab monotherapy may delay SMM progression to MM, as most pts achieved a best overall response of SD or MR, with ≥MR in 29% of pts, including PR in 10%, and favorable PFS. Treatment was generally well tolerated; the AE profile was consistent with prior elotuzumab experience. Funding: BMS. Medical writing: S Addison, Caudex, funded by BMS. 1. Hsi E et al. Clin Cancer Res 2008;14:2775–84
2. Kyle R et. al. Leukemia 2010;24:1121–27

Session topic: Experimental approaches for plasma cell disorders
Keyword(s): Monoclonal antibody, Multiple myeloma, Smoldering
{{ help_message }}
{{filter}}


