QUANTITATIVE MRD IS PROGNOSTIC FOR PROGRESSION-FREE & OVERALL SURVIVAL IN ELDERLY PATIENTS RECEIVING CHLORAMBUCIL ALONE OR WITH OBINUTUZUMAB/RITUXIMAB: A PROSPECTIVE ANALYSIS OF THE GCLLSG CLL11 STUDY
(Abstract release date: 05/19/16)
EHA Library. Ritgen M. 06/11/16; 135184; S428

Dr. Matthias Ritgen
Contributions
Contributions
Abstract
Abstract: S428
Type: Oral Presentation
Presentation during EHA21: On Saturday, June 11, 2016 from 11:45 - 12:00
Location: Hall A1
Background
The CLL11 study (NCT01010061) is an ongoing open-label, randomized, three-arm study in patients (pts) with previously untreated chronic lymphocytic leukemia (CLL), comparing the efficacy and safety of obinutuzumab (GA101; GAZYVA/GAZYVARO; G) plus chlorambucil (Clb; G-Clb) with rituximab (R) plus Clb (R-Clb) or Clb alone.
Aims
To determine the prognostic value of minimal residual disease (MRD) quantification with respect to clinical risk factors, treatment regimen, and MRD-source tissue in an advanced-age CLL population.
Methods
In total, 781 patients with treatment-naïve CLL, a median age of 73 years and a Cumulative Illness Rating Scale total score of >6 were included and randomized to receive Clb, G-Clb or R-Clb. Peripheral blood (PB) samples were taken at repeated timepoints during and up to 12 months (mo) after treatment. Bone marrow (BM) aspirate was taken 3 mo after end of treatment (EOT) to confirm complete response (CR). MRD was analyzed by quantitative immunoglobulin allele-specific real-time PCR. MRD results at EOT (PB or BM) were available in 73% of pts. Outcome was analyzed according to known MRD risk groups, i.e. MRD-positive (+ve; ≥1%), MRD intermediate (int; <1% and ≥0.01%) and MRD-negative (-ve; <0.01%), and other known risk factors.
Results
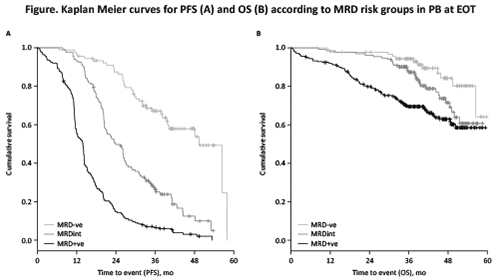
At EOT, combination treatment with either G-Clb or R-Clb achieved higher MRD negativity rates in PB and BM compared with Clb alone (PB: G-Clb 35.8% vs R-Clb 3.3% vs Clb 0%; BM: G-Clb 18.2% vs R-Clb 2.6% vs Clb 0%). Due to the absence of MRD negativity in the Clb arm, all further MRD analyses were restricted to the comparison of G-Clb vs R-Clb. When measured in PB, MRD risk groups significantly correlated with progression-free survival (PFS; median PFS: MRD-ve 49.3 mo; MRDint 23.9 mo; MRD+ve 13.9 mo; p<0.001) and overall survival (OS; median OS not reached in any group; p<0.001; see Figure for KM curves). When MRD was measured in PB, three-year OS rates were: MRD-ve 94.3%; MRDint 87.3%; and MRD+ve 69.5%. When comparing G-Clb with R-Clb, median PFS in the MRD-ve group was not significantly different (p=0.15). Results were similar for MRD measured in PB after 3 cycles of treatment (interim staging [IST]; median PFS: MRD-ve not reached; MRDint 27.3 mo; MRD+ve 15.2 mo; p<0.001). A total of 53/417 (12.7%) pts converted from PB MRD+ve or MRDint at IST to MRD-ve at EOT across the G-Clb/R-Clb arms. In multivariate analysis (including various baseline markers), MRD in PB at EOT was an independent prognostic factor for PFS (HR 5.29, 95% CI 3.48-8.04, p<0.001) and OS (HR 3.04, 95% CI 1.53-6.03, p=0.002), with the highest weight in the model. When analyzing MRD in PB at EOT in conjunction with clinical remission status, MRD-ve/CR and MRD-ve/PR pts had similar PFS (median PFS 49.3 mo vs 56.4 mo, HR 1.74, 95% CI 0.79-3.86, p=0.17), whereas MRD+ve/CR pts had a worse outcome than MRD-ve/CR pts (median PFS 32.7 mo vs 49.3 mo, HR 3.6, 95% CI 1.64-7.99, p=0.001).
Conclusion
Data presented are consistent with the CLL8 study and confirm that, when measured in PB or BM, MRD is prognostic in elderly pts with comorbidities, with G-Clb achieving a much higher rate of MRD negativity than R-Clb and Clb alone. In this cohort, pts achieving MRD-ve status at IST had a similar outcome to pts with later conversion (at EOT). Although MRD measurement in BM seems to be more sensitive, MRD measurement in PB, at least in this trial, is sufficient to identify MRD based risk groups.
Session topic: Innovative therapies in CLL
Keyword(s): Chronic lymphocytic leukemia, Minimal residual disease (MRD), Obinutuzumab, Rituximab
Type: Oral Presentation
Presentation during EHA21: On Saturday, June 11, 2016 from 11:45 - 12:00
Location: Hall A1
Background
The CLL11 study (NCT01010061) is an ongoing open-label, randomized, three-arm study in patients (pts) with previously untreated chronic lymphocytic leukemia (CLL), comparing the efficacy and safety of obinutuzumab (GA101; GAZYVA/GAZYVARO; G) plus chlorambucil (Clb; G-Clb) with rituximab (R) plus Clb (R-Clb) or Clb alone.
Aims
To determine the prognostic value of minimal residual disease (MRD) quantification with respect to clinical risk factors, treatment regimen, and MRD-source tissue in an advanced-age CLL population.
Methods
In total, 781 patients with treatment-naïve CLL, a median age of 73 years and a Cumulative Illness Rating Scale total score of >6 were included and randomized to receive Clb, G-Clb or R-Clb. Peripheral blood (PB) samples were taken at repeated timepoints during and up to 12 months (mo) after treatment. Bone marrow (BM) aspirate was taken 3 mo after end of treatment (EOT) to confirm complete response (CR). MRD was analyzed by quantitative immunoglobulin allele-specific real-time PCR. MRD results at EOT (PB or BM) were available in 73% of pts. Outcome was analyzed according to known MRD risk groups, i.e. MRD-positive (+ve; ≥1%), MRD intermediate (int; <1% and ≥0.01%) and MRD-negative (-ve; <0.01%), and other known risk factors.
Results
At EOT, combination treatment with either G-Clb or R-Clb achieved higher MRD negativity rates in PB and BM compared with Clb alone (PB: G-Clb 35.8% vs R-Clb 3.3% vs Clb 0%; BM: G-Clb 18.2% vs R-Clb 2.6% vs Clb 0%). Due to the absence of MRD negativity in the Clb arm, all further MRD analyses were restricted to the comparison of G-Clb vs R-Clb. When measured in PB, MRD risk groups significantly correlated with progression-free survival (PFS; median PFS: MRD-ve 49.3 mo; MRDint 23.9 mo; MRD+ve 13.9 mo; p<0.001) and overall survival (OS; median OS not reached in any group; p<0.001; see Figure for KM curves). When MRD was measured in PB, three-year OS rates were: MRD-ve 94.3%; MRDint 87.3%; and MRD+ve 69.5%. When comparing G-Clb with R-Clb, median PFS in the MRD-ve group was not significantly different (p=0.15). Results were similar for MRD measured in PB after 3 cycles of treatment (interim staging [IST]; median PFS: MRD-ve not reached; MRDint 27.3 mo; MRD+ve 15.2 mo; p<0.001). A total of 53/417 (12.7%) pts converted from PB MRD+ve or MRDint at IST to MRD-ve at EOT across the G-Clb/R-Clb arms. In multivariate analysis (including various baseline markers), MRD in PB at EOT was an independent prognostic factor for PFS (HR 5.29, 95% CI 3.48-8.04, p<0.001) and OS (HR 3.04, 95% CI 1.53-6.03, p=0.002), with the highest weight in the model. When analyzing MRD in PB at EOT in conjunction with clinical remission status, MRD-ve/CR and MRD-ve/PR pts had similar PFS (median PFS 49.3 mo vs 56.4 mo, HR 1.74, 95% CI 0.79-3.86, p=0.17), whereas MRD+ve/CR pts had a worse outcome than MRD-ve/CR pts (median PFS 32.7 mo vs 49.3 mo, HR 3.6, 95% CI 1.64-7.99, p=0.001).
Conclusion
Data presented are consistent with the CLL8 study and confirm that, when measured in PB or BM, MRD is prognostic in elderly pts with comorbidities, with G-Clb achieving a much higher rate of MRD negativity than R-Clb and Clb alone. In this cohort, pts achieving MRD-ve status at IST had a similar outcome to pts with later conversion (at EOT). Although MRD measurement in BM seems to be more sensitive, MRD measurement in PB, at least in this trial, is sufficient to identify MRD based risk groups.

Session topic: Innovative therapies in CLL
Keyword(s): Chronic lymphocytic leukemia, Minimal residual disease (MRD), Obinutuzumab, Rituximab
Abstract: S428
Type: Oral Presentation
Presentation during EHA21: On Saturday, June 11, 2016 from 11:45 - 12:00
Location: Hall A1
Background
The CLL11 study (NCT01010061) is an ongoing open-label, randomized, three-arm study in patients (pts) with previously untreated chronic lymphocytic leukemia (CLL), comparing the efficacy and safety of obinutuzumab (GA101; GAZYVA/GAZYVARO; G) plus chlorambucil (Clb; G-Clb) with rituximab (R) plus Clb (R-Clb) or Clb alone.
Aims
To determine the prognostic value of minimal residual disease (MRD) quantification with respect to clinical risk factors, treatment regimen, and MRD-source tissue in an advanced-age CLL population.
Methods
In total, 781 patients with treatment-naïve CLL, a median age of 73 years and a Cumulative Illness Rating Scale total score of >6 were included and randomized to receive Clb, G-Clb or R-Clb. Peripheral blood (PB) samples were taken at repeated timepoints during and up to 12 months (mo) after treatment. Bone marrow (BM) aspirate was taken 3 mo after end of treatment (EOT) to confirm complete response (CR). MRD was analyzed by quantitative immunoglobulin allele-specific real-time PCR. MRD results at EOT (PB or BM) were available in 73% of pts. Outcome was analyzed according to known MRD risk groups, i.e. MRD-positive (+ve; ≥1%), MRD intermediate (int; <1% and ≥0.01%) and MRD-negative (-ve; <0.01%), and other known risk factors.
Results
At EOT, combination treatment with either G-Clb or R-Clb achieved higher MRD negativity rates in PB and BM compared with Clb alone (PB: G-Clb 35.8% vs R-Clb 3.3% vs Clb 0%; BM: G-Clb 18.2% vs R-Clb 2.6% vs Clb 0%). Due to the absence of MRD negativity in the Clb arm, all further MRD analyses were restricted to the comparison of G-Clb vs R-Clb. When measured in PB, MRD risk groups significantly correlated with progression-free survival (PFS; median PFS: MRD-ve 49.3 mo; MRDint 23.9 mo; MRD+ve 13.9 mo; p<0.001) and overall survival (OS; median OS not reached in any group; p<0.001; see Figure for KM curves). When MRD was measured in PB, three-year OS rates were: MRD-ve 94.3%; MRDint 87.3%; and MRD+ve 69.5%. When comparing G-Clb with R-Clb, median PFS in the MRD-ve group was not significantly different (p=0.15). Results were similar for MRD measured in PB after 3 cycles of treatment (interim staging [IST]; median PFS: MRD-ve not reached; MRDint 27.3 mo; MRD+ve 15.2 mo; p<0.001). A total of 53/417 (12.7%) pts converted from PB MRD+ve or MRDint at IST to MRD-ve at EOT across the G-Clb/R-Clb arms. In multivariate analysis (including various baseline markers), MRD in PB at EOT was an independent prognostic factor for PFS (HR 5.29, 95% CI 3.48-8.04, p<0.001) and OS (HR 3.04, 95% CI 1.53-6.03, p=0.002), with the highest weight in the model. When analyzing MRD in PB at EOT in conjunction with clinical remission status, MRD-ve/CR and MRD-ve/PR pts had similar PFS (median PFS 49.3 mo vs 56.4 mo, HR 1.74, 95% CI 0.79-3.86, p=0.17), whereas MRD+ve/CR pts had a worse outcome than MRD-ve/CR pts (median PFS 32.7 mo vs 49.3 mo, HR 3.6, 95% CI 1.64-7.99, p=0.001).
Conclusion
Data presented are consistent with the CLL8 study and confirm that, when measured in PB or BM, MRD is prognostic in elderly pts with comorbidities, with G-Clb achieving a much higher rate of MRD negativity than R-Clb and Clb alone. In this cohort, pts achieving MRD-ve status at IST had a similar outcome to pts with later conversion (at EOT). Although MRD measurement in BM seems to be more sensitive, MRD measurement in PB, at least in this trial, is sufficient to identify MRD based risk groups.

Session topic: Innovative therapies in CLL
Keyword(s): Chronic lymphocytic leukemia, Minimal residual disease (MRD), Obinutuzumab, Rituximab
Type: Oral Presentation
Presentation during EHA21: On Saturday, June 11, 2016 from 11:45 - 12:00
Location: Hall A1
Background
The CLL11 study (NCT01010061) is an ongoing open-label, randomized, three-arm study in patients (pts) with previously untreated chronic lymphocytic leukemia (CLL), comparing the efficacy and safety of obinutuzumab (GA101; GAZYVA/GAZYVARO; G) plus chlorambucil (Clb; G-Clb) with rituximab (R) plus Clb (R-Clb) or Clb alone.
Aims
To determine the prognostic value of minimal residual disease (MRD) quantification with respect to clinical risk factors, treatment regimen, and MRD-source tissue in an advanced-age CLL population.
Methods
In total, 781 patients with treatment-naïve CLL, a median age of 73 years and a Cumulative Illness Rating Scale total score of >6 were included and randomized to receive Clb, G-Clb or R-Clb. Peripheral blood (PB) samples were taken at repeated timepoints during and up to 12 months (mo) after treatment. Bone marrow (BM) aspirate was taken 3 mo after end of treatment (EOT) to confirm complete response (CR). MRD was analyzed by quantitative immunoglobulin allele-specific real-time PCR. MRD results at EOT (PB or BM) were available in 73% of pts. Outcome was analyzed according to known MRD risk groups, i.e. MRD-positive (+ve; ≥1%), MRD intermediate (int; <1% and ≥0.01%) and MRD-negative (-ve; <0.01%), and other known risk factors.
Results
At EOT, combination treatment with either G-Clb or R-Clb achieved higher MRD negativity rates in PB and BM compared with Clb alone (PB: G-Clb 35.8% vs R-Clb 3.3% vs Clb 0%; BM: G-Clb 18.2% vs R-Clb 2.6% vs Clb 0%). Due to the absence of MRD negativity in the Clb arm, all further MRD analyses were restricted to the comparison of G-Clb vs R-Clb. When measured in PB, MRD risk groups significantly correlated with progression-free survival (PFS; median PFS: MRD-ve 49.3 mo; MRDint 23.9 mo; MRD+ve 13.9 mo; p<0.001) and overall survival (OS; median OS not reached in any group; p<0.001; see Figure for KM curves). When MRD was measured in PB, three-year OS rates were: MRD-ve 94.3%; MRDint 87.3%; and MRD+ve 69.5%. When comparing G-Clb with R-Clb, median PFS in the MRD-ve group was not significantly different (p=0.15). Results were similar for MRD measured in PB after 3 cycles of treatment (interim staging [IST]; median PFS: MRD-ve not reached; MRDint 27.3 mo; MRD+ve 15.2 mo; p<0.001). A total of 53/417 (12.7%) pts converted from PB MRD+ve or MRDint at IST to MRD-ve at EOT across the G-Clb/R-Clb arms. In multivariate analysis (including various baseline markers), MRD in PB at EOT was an independent prognostic factor for PFS (HR 5.29, 95% CI 3.48-8.04, p<0.001) and OS (HR 3.04, 95% CI 1.53-6.03, p=0.002), with the highest weight in the model. When analyzing MRD in PB at EOT in conjunction with clinical remission status, MRD-ve/CR and MRD-ve/PR pts had similar PFS (median PFS 49.3 mo vs 56.4 mo, HR 1.74, 95% CI 0.79-3.86, p=0.17), whereas MRD+ve/CR pts had a worse outcome than MRD-ve/CR pts (median PFS 32.7 mo vs 49.3 mo, HR 3.6, 95% CI 1.64-7.99, p=0.001).
Conclusion
Data presented are consistent with the CLL8 study and confirm that, when measured in PB or BM, MRD is prognostic in elderly pts with comorbidities, with G-Clb achieving a much higher rate of MRD negativity than R-Clb and Clb alone. In this cohort, pts achieving MRD-ve status at IST had a similar outcome to pts with later conversion (at EOT). Although MRD measurement in BM seems to be more sensitive, MRD measurement in PB, at least in this trial, is sufficient to identify MRD based risk groups.

Session topic: Innovative therapies in CLL
Keyword(s): Chronic lymphocytic leukemia, Minimal residual disease (MRD), Obinutuzumab, Rituximab
{{ help_message }}
{{filter}}


