REAL-WORLD PRESCRIBING PATTERNS IN U.S. MULTIPLE MYELOMA (MM) PATIENTS REFRACTORY TO LENALIDOMIDE IN THE FRONT-LINE
(Abstract release date: 05/19/16)
EHA Library. Jhaveri M. 06/09/16; 132861; E1312

Dr. Mehul Jhaveri
Contributions
Contributions
Abstract
Abstract: E1312
Type: Eposter Presentation
Background
The introduction of novel agents into the treatment paradigm of MM has improved outcomes; however, MM remains incurable with patients requiring subsequent lines of therapy post-relapse. Patients with relapsed MM who are refractory to bortezomib and refractory to or ineligible for lenalidomide have a median overall and event-free survival of 9 and 5 months, respectively (Kumar 2012). There is a scarcity of data regarding real-world treatment patterns and clinical outcomes in previous lenalidomide-exposed patients with relapsed / refractory MM (RRMM).
Aims
This study aims to describe the patient characteristics and treatment patterns among MM patients treated in the real-world who relapsed or were refractory to lenalidomide in first-line treatment (1LT) within the U.S.
Methods
This was a retrospective cohort study using a large national EMR database. Newly diagnosed MM patients initiating a lenalidomide-based 1LT between 1/2008 and 12/2014 were followed 1 year prior to and up to 7 years after diagnosis. Maintenance therapy, if given, was included within 1LT. Patients were required to be ≥18 years of age with evidence of starting second-line therapy (2LT), which was identified accordingly: 1) retreatment after a treatment gap of >3 months of 1LT, or 2) a switch to another drug combination after starting 1LT. All patients were followed until death/loss to follow up or the end of study period (6/30/2015). Based on the International Myeloma Workshop Consensus Panel 1 and using initiation of 2LT as a surrogate marker for non-response or progressive disease, lenalidomide-refractory patients, for the purpose of this study, were defined as those who initiated 2LT within 60 days of 1LT discontinuation (Rajkumar 2011). Relapsed patients were those with a >60 day gap between end of 1LT and 2LT initiation.
Results
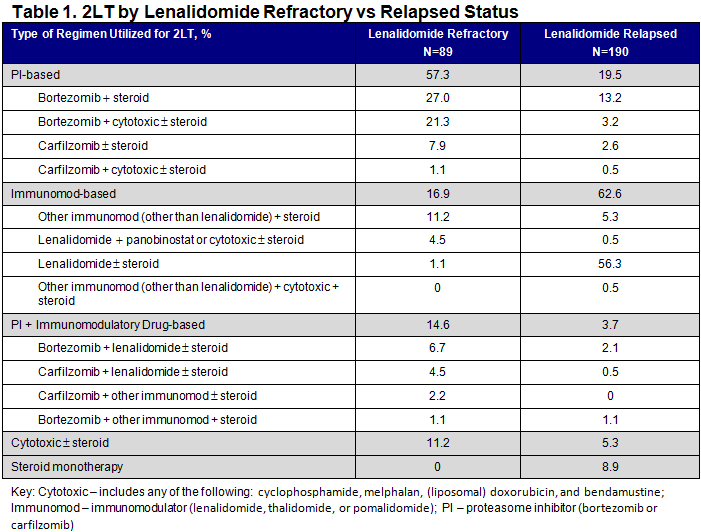
There were 279 RRMM patients who received lenalidomide in 1LT (n=89 (31.9%) refractory; n=190 (68.1%) relapsed); overall, mean age was 69.9 years (standard deviation (SD): 9.9) and 49.5% were male. More patients in the refractory population had known high cytogenetic risk disease (presence of any: del[17p], t[4:14], t[14:16]) than in the relapsed population (16.9% vs 3.2%), and refractory patients were more likely to have renal impairment (52.8% vs 37.9%), anemia (65.2% vs 45.3%), hypercalcemia (15.7% vs 3.7%) and bone disease (30.3% vs 23.7%) at initiation of 2LT than relapsed patients. Refractory patients were more likely than relapsed patients to receive only PI-based therapy (57.3% vs 19.5%) and less likely to receive only immunomodulatory drug (immunomod)-based therapy (16.9% vs 62.6%) in 2LT (P<0.001 for both; chi-square test). In addition, use of ≥3 drug-therapy in 2LT was higher in the refractory population than in the relapsed population (40.5% vs 8.4%) (P<0.001; chi-square test).
Conclusion
RRMM patients refractory to lenalidomide in 1LT presented with a higher disease-related symptom burden at start of 2LT, were treated more aggressively in 2LT, and were more likely to switch to PI-based therapy than those defined as relapsed. One limitation to this real-world study is that patients may have stopped 1LT due to factors other than nonresponse or progressive disease.References: Kumar et al. Leukemia. 2012 January; 26(1): 149–157; Rajkumar et al. Blood. 2011;117(18):4691-4695.
Session topic: E-poster
Keyword(s): Myeloma, Refractory, Relapse, Treatment
Type: Eposter Presentation
Background
The introduction of novel agents into the treatment paradigm of MM has improved outcomes; however, MM remains incurable with patients requiring subsequent lines of therapy post-relapse. Patients with relapsed MM who are refractory to bortezomib and refractory to or ineligible for lenalidomide have a median overall and event-free survival of 9 and 5 months, respectively (Kumar 2012). There is a scarcity of data regarding real-world treatment patterns and clinical outcomes in previous lenalidomide-exposed patients with relapsed / refractory MM (RRMM).
Aims
This study aims to describe the patient characteristics and treatment patterns among MM patients treated in the real-world who relapsed or were refractory to lenalidomide in first-line treatment (1LT) within the U.S.
Methods
This was a retrospective cohort study using a large national EMR database. Newly diagnosed MM patients initiating a lenalidomide-based 1LT between 1/2008 and 12/2014 were followed 1 year prior to and up to 7 years after diagnosis. Maintenance therapy, if given, was included within 1LT. Patients were required to be ≥18 years of age with evidence of starting second-line therapy (2LT), which was identified accordingly: 1) retreatment after a treatment gap of >3 months of 1LT, or 2) a switch to another drug combination after starting 1LT. All patients were followed until death/loss to follow up or the end of study period (6/30/2015). Based on the International Myeloma Workshop Consensus Panel 1 and using initiation of 2LT as a surrogate marker for non-response or progressive disease, lenalidomide-refractory patients, for the purpose of this study, were defined as those who initiated 2LT within 60 days of 1LT discontinuation (Rajkumar 2011). Relapsed patients were those with a >60 day gap between end of 1LT and 2LT initiation.
Results
There were 279 RRMM patients who received lenalidomide in 1LT (n=89 (31.9%) refractory; n=190 (68.1%) relapsed); overall, mean age was 69.9 years (standard deviation (SD): 9.9) and 49.5% were male. More patients in the refractory population had known high cytogenetic risk disease (presence of any: del[17p], t[4:14], t[14:16]) than in the relapsed population (16.9% vs 3.2%), and refractory patients were more likely to have renal impairment (52.8% vs 37.9%), anemia (65.2% vs 45.3%), hypercalcemia (15.7% vs 3.7%) and bone disease (30.3% vs 23.7%) at initiation of 2LT than relapsed patients. Refractory patients were more likely than relapsed patients to receive only PI-based therapy (57.3% vs 19.5%) and less likely to receive only immunomodulatory drug (immunomod)-based therapy (16.9% vs 62.6%) in 2LT (P<0.001 for both; chi-square test). In addition, use of ≥3 drug-therapy in 2LT was higher in the refractory population than in the relapsed population (40.5% vs 8.4%) (P<0.001; chi-square test).
Conclusion
RRMM patients refractory to lenalidomide in 1LT presented with a higher disease-related symptom burden at start of 2LT, were treated more aggressively in 2LT, and were more likely to switch to PI-based therapy than those defined as relapsed. One limitation to this real-world study is that patients may have stopped 1LT due to factors other than nonresponse or progressive disease.References: Kumar et al. Leukemia. 2012 January; 26(1): 149–157; Rajkumar et al. Blood. 2011;117(18):4691-4695.

Session topic: E-poster
Keyword(s): Myeloma, Refractory, Relapse, Treatment
Abstract: E1312
Type: Eposter Presentation
Background
The introduction of novel agents into the treatment paradigm of MM has improved outcomes; however, MM remains incurable with patients requiring subsequent lines of therapy post-relapse. Patients with relapsed MM who are refractory to bortezomib and refractory to or ineligible for lenalidomide have a median overall and event-free survival of 9 and 5 months, respectively (Kumar 2012). There is a scarcity of data regarding real-world treatment patterns and clinical outcomes in previous lenalidomide-exposed patients with relapsed / refractory MM (RRMM).
Aims
This study aims to describe the patient characteristics and treatment patterns among MM patients treated in the real-world who relapsed or were refractory to lenalidomide in first-line treatment (1LT) within the U.S.
Methods
This was a retrospective cohort study using a large national EMR database. Newly diagnosed MM patients initiating a lenalidomide-based 1LT between 1/2008 and 12/2014 were followed 1 year prior to and up to 7 years after diagnosis. Maintenance therapy, if given, was included within 1LT. Patients were required to be ≥18 years of age with evidence of starting second-line therapy (2LT), which was identified accordingly: 1) retreatment after a treatment gap of >3 months of 1LT, or 2) a switch to another drug combination after starting 1LT. All patients were followed until death/loss to follow up or the end of study period (6/30/2015). Based on the International Myeloma Workshop Consensus Panel 1 and using initiation of 2LT as a surrogate marker for non-response or progressive disease, lenalidomide-refractory patients, for the purpose of this study, were defined as those who initiated 2LT within 60 days of 1LT discontinuation (Rajkumar 2011). Relapsed patients were those with a >60 day gap between end of 1LT and 2LT initiation.
Results
There were 279 RRMM patients who received lenalidomide in 1LT (n=89 (31.9%) refractory; n=190 (68.1%) relapsed); overall, mean age was 69.9 years (standard deviation (SD): 9.9) and 49.5% were male. More patients in the refractory population had known high cytogenetic risk disease (presence of any: del[17p], t[4:14], t[14:16]) than in the relapsed population (16.9% vs 3.2%), and refractory patients were more likely to have renal impairment (52.8% vs 37.9%), anemia (65.2% vs 45.3%), hypercalcemia (15.7% vs 3.7%) and bone disease (30.3% vs 23.7%) at initiation of 2LT than relapsed patients. Refractory patients were more likely than relapsed patients to receive only PI-based therapy (57.3% vs 19.5%) and less likely to receive only immunomodulatory drug (immunomod)-based therapy (16.9% vs 62.6%) in 2LT (P<0.001 for both; chi-square test). In addition, use of ≥3 drug-therapy in 2LT was higher in the refractory population than in the relapsed population (40.5% vs 8.4%) (P<0.001; chi-square test).
Conclusion
RRMM patients refractory to lenalidomide in 1LT presented with a higher disease-related symptom burden at start of 2LT, were treated more aggressively in 2LT, and were more likely to switch to PI-based therapy than those defined as relapsed. One limitation to this real-world study is that patients may have stopped 1LT due to factors other than nonresponse or progressive disease.References: Kumar et al. Leukemia. 2012 January; 26(1): 149–157; Rajkumar et al. Blood. 2011;117(18):4691-4695.

Session topic: E-poster
Keyword(s): Myeloma, Refractory, Relapse, Treatment
Type: Eposter Presentation
Background
The introduction of novel agents into the treatment paradigm of MM has improved outcomes; however, MM remains incurable with patients requiring subsequent lines of therapy post-relapse. Patients with relapsed MM who are refractory to bortezomib and refractory to or ineligible for lenalidomide have a median overall and event-free survival of 9 and 5 months, respectively (Kumar 2012). There is a scarcity of data regarding real-world treatment patterns and clinical outcomes in previous lenalidomide-exposed patients with relapsed / refractory MM (RRMM).
Aims
This study aims to describe the patient characteristics and treatment patterns among MM patients treated in the real-world who relapsed or were refractory to lenalidomide in first-line treatment (1LT) within the U.S.
Methods
This was a retrospective cohort study using a large national EMR database. Newly diagnosed MM patients initiating a lenalidomide-based 1LT between 1/2008 and 12/2014 were followed 1 year prior to and up to 7 years after diagnosis. Maintenance therapy, if given, was included within 1LT. Patients were required to be ≥18 years of age with evidence of starting second-line therapy (2LT), which was identified accordingly: 1) retreatment after a treatment gap of >3 months of 1LT, or 2) a switch to another drug combination after starting 1LT. All patients were followed until death/loss to follow up or the end of study period (6/30/2015). Based on the International Myeloma Workshop Consensus Panel 1 and using initiation of 2LT as a surrogate marker for non-response or progressive disease, lenalidomide-refractory patients, for the purpose of this study, were defined as those who initiated 2LT within 60 days of 1LT discontinuation (Rajkumar 2011). Relapsed patients were those with a >60 day gap between end of 1LT and 2LT initiation.
Results
There were 279 RRMM patients who received lenalidomide in 1LT (n=89 (31.9%) refractory; n=190 (68.1%) relapsed); overall, mean age was 69.9 years (standard deviation (SD): 9.9) and 49.5% were male. More patients in the refractory population had known high cytogenetic risk disease (presence of any: del[17p], t[4:14], t[14:16]) than in the relapsed population (16.9% vs 3.2%), and refractory patients were more likely to have renal impairment (52.8% vs 37.9%), anemia (65.2% vs 45.3%), hypercalcemia (15.7% vs 3.7%) and bone disease (30.3% vs 23.7%) at initiation of 2LT than relapsed patients. Refractory patients were more likely than relapsed patients to receive only PI-based therapy (57.3% vs 19.5%) and less likely to receive only immunomodulatory drug (immunomod)-based therapy (16.9% vs 62.6%) in 2LT (P<0.001 for both; chi-square test). In addition, use of ≥3 drug-therapy in 2LT was higher in the refractory population than in the relapsed population (40.5% vs 8.4%) (P<0.001; chi-square test).
Conclusion
RRMM patients refractory to lenalidomide in 1LT presented with a higher disease-related symptom burden at start of 2LT, were treated more aggressively in 2LT, and were more likely to switch to PI-based therapy than those defined as relapsed. One limitation to this real-world study is that patients may have stopped 1LT due to factors other than nonresponse or progressive disease.References: Kumar et al. Leukemia. 2012 January; 26(1): 149–157; Rajkumar et al. Blood. 2011;117(18):4691-4695.

Session topic: E-poster
Keyword(s): Myeloma, Refractory, Relapse, Treatment
{{ help_message }}
{{filter}}


