DURATION OF THERAPY IN U.S. PATIENTS TREATED FOR RELAPSED / REFRACTORY MULTIPLE MYELOMA (RRMM) IN THE REAL-WORLD
(Abstract release date: 05/19/16)
EHA Library. Romanus D. 06/09/16; 132850; E1301

Dr. Dorothy Romanus
Contributions
Contributions
Abstract
Abstract: E1301
Type: Eposter Presentation
Background
Extended duration of therapy (DOT) is associated with better clinical outcomes in clinical trials in patients (pts) with multiple myeloma (MM) (Palumbo 2015; Mateos 2015). Many of the factors that influence DOT are more prevalent outside of clinical trials, including older age, and concomitant comorbidities. Real-world data related to DOT among RRMM pts are limited.
Aims
This study aims to 1) describe DOT of second line (2LT) and third line (3LT) therapy in RRMM, and 2) evaluate the associations between 2LT DOT and age; comorbidities; and regimen type.
Methods
In this retrospective cohort study, newly diagnosed adult MM pts initiating first-line therapy (1LT) between 1/2008 and 12/2014 were followed in a large U.S. national electronic medical records (EMR) database to identify 2LT accordingly: 1) retreatment after a treatment gap of >3 months of 1LT, or 2) a switch to another drug combination after starting 1LT. 3LT began with a switch to another regimen after 2LT. Pts with salvage stem cell transplants were excluded. Kaplan-Meier analyses were performed to calculate DOT from start of both 2LT and 3LT. Observations were censored at time of loss to follow up/end of study period (6/30/2015). A two-tailed log-rank test was used to test for statistical significance between groups.
Results
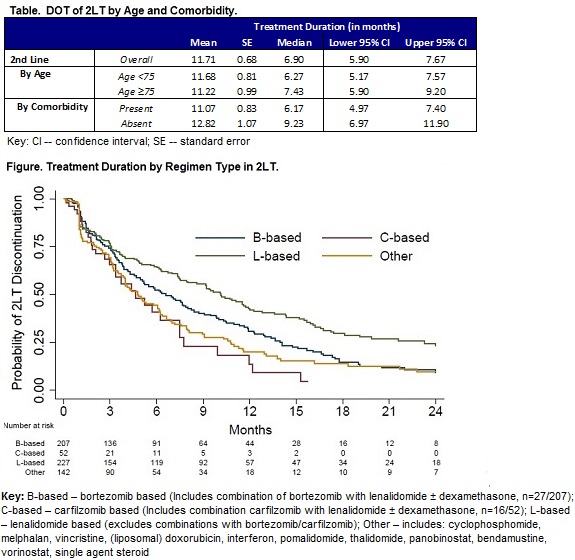
Among 628 pts, 37.1% were ≥75 years of age; 51.0% were male; 66.4% had ≥1 of the following comorbidities of interest at initiation of 2LT: diabetes, renal insufficiency, thromboembolic disease, cardiovascular disease, peripheral neuropathy. In 2LT, pts were most commonly treated with lenalidomide (L)-based regimens (36.2%) without bortezomib (B), followed by B-based therapies (33.0%, with/without L). Carfilzomib (C)-based treatments were least common (8.3%, with/without L). The remaining regimens (Other) comprised of MM-therapies without L, B, or C (22.6%). Overall, the median DOT in 2LT and 3LT was 6.9 months (mos) (95% CI: 5.9, 7.7) and 5.5 mos (95% CI: 4.0, 6.2), respectively. DOT in 2LT was similar for those <75 vs ≥75 years, median: 6.3 mos (95% CI: 5.2, 7.6) and 7.4 mos (95% CI: 5.9, 9.2), P>0.05, respectively (Table). Pts with baseline comorbidities of interest were significantly more likely to have a shorter DOT in 2LT, median: 6.2 mos (95% CI: 5.0, 7.4) compared to pts without, median: 9.2 mos (95% CI: 7.0, 11.9), P=0.02. In 2LT, the drug composition of the regimen was significantly associated with DOT (P<0.0001). L-based therapies had the longest DOT (median: 10.1 mos; 95% CI: 7.9, 11.9) compared to B-based (median: 6.6 mos; 95% CI: 5.1, 8.0), C-based (median: 4.6 mos; 95% CI: 3.0, 7.5) and Other regimens (median: 4.8 mos; 95% CI: 3.9, 6.2) (Figure).
Conclusion
DOT wanes with subsequent lines of therapy. Baseline comorbidities and regimen type in 2LT are significantly associated with DOT. The longest median DOT was observed with L-based therapies and the shortest DOT with C-based therapies. Tailored therapy choices that account for pts’ baseline comorbidities may mitigate the observed variation in DOT.References: Palumbo et al. JCO 2015;33:3459-66.Mateos et al. Am J Hematol.2015;90:314-9.
Session topic: E-poster
Keyword(s): Myeloma, Refractory, Relapse, Treatment
Type: Eposter Presentation
Background
Extended duration of therapy (DOT) is associated with better clinical outcomes in clinical trials in patients (pts) with multiple myeloma (MM) (Palumbo 2015; Mateos 2015). Many of the factors that influence DOT are more prevalent outside of clinical trials, including older age, and concomitant comorbidities. Real-world data related to DOT among RRMM pts are limited.
Aims
This study aims to 1) describe DOT of second line (2LT) and third line (3LT) therapy in RRMM, and 2) evaluate the associations between 2LT DOT and age; comorbidities; and regimen type.
Methods
In this retrospective cohort study, newly diagnosed adult MM pts initiating first-line therapy (1LT) between 1/2008 and 12/2014 were followed in a large U.S. national electronic medical records (EMR) database to identify 2LT accordingly: 1) retreatment after a treatment gap of >3 months of 1LT, or 2) a switch to another drug combination after starting 1LT. 3LT began with a switch to another regimen after 2LT. Pts with salvage stem cell transplants were excluded. Kaplan-Meier analyses were performed to calculate DOT from start of both 2LT and 3LT. Observations were censored at time of loss to follow up/end of study period (6/30/2015). A two-tailed log-rank test was used to test for statistical significance between groups.
Results
Among 628 pts, 37.1% were ≥75 years of age; 51.0% were male; 66.4% had ≥1 of the following comorbidities of interest at initiation of 2LT: diabetes, renal insufficiency, thromboembolic disease, cardiovascular disease, peripheral neuropathy. In 2LT, pts were most commonly treated with lenalidomide (L)-based regimens (36.2%) without bortezomib (B), followed by B-based therapies (33.0%, with/without L). Carfilzomib (C)-based treatments were least common (8.3%, with/without L). The remaining regimens (Other) comprised of MM-therapies without L, B, or C (22.6%). Overall, the median DOT in 2LT and 3LT was 6.9 months (mos) (95% CI: 5.9, 7.7) and 5.5 mos (95% CI: 4.0, 6.2), respectively. DOT in 2LT was similar for those <75 vs ≥75 years, median: 6.3 mos (95% CI: 5.2, 7.6) and 7.4 mos (95% CI: 5.9, 9.2), P>0.05, respectively (Table). Pts with baseline comorbidities of interest were significantly more likely to have a shorter DOT in 2LT, median: 6.2 mos (95% CI: 5.0, 7.4) compared to pts without, median: 9.2 mos (95% CI: 7.0, 11.9), P=0.02. In 2LT, the drug composition of the regimen was significantly associated with DOT (P<0.0001). L-based therapies had the longest DOT (median: 10.1 mos; 95% CI: 7.9, 11.9) compared to B-based (median: 6.6 mos; 95% CI: 5.1, 8.0), C-based (median: 4.6 mos; 95% CI: 3.0, 7.5) and Other regimens (median: 4.8 mos; 95% CI: 3.9, 6.2) (Figure).
Conclusion
DOT wanes with subsequent lines of therapy. Baseline comorbidities and regimen type in 2LT are significantly associated with DOT. The longest median DOT was observed with L-based therapies and the shortest DOT with C-based therapies. Tailored therapy choices that account for pts’ baseline comorbidities may mitigate the observed variation in DOT.References: Palumbo et al. JCO 2015;33:3459-66.Mateos et al. Am J Hematol.2015;90:314-9.

Session topic: E-poster
Keyword(s): Myeloma, Refractory, Relapse, Treatment
Abstract: E1301
Type: Eposter Presentation
Background
Extended duration of therapy (DOT) is associated with better clinical outcomes in clinical trials in patients (pts) with multiple myeloma (MM) (Palumbo 2015; Mateos 2015). Many of the factors that influence DOT are more prevalent outside of clinical trials, including older age, and concomitant comorbidities. Real-world data related to DOT among RRMM pts are limited.
Aims
This study aims to 1) describe DOT of second line (2LT) and third line (3LT) therapy in RRMM, and 2) evaluate the associations between 2LT DOT and age; comorbidities; and regimen type.
Methods
In this retrospective cohort study, newly diagnosed adult MM pts initiating first-line therapy (1LT) between 1/2008 and 12/2014 were followed in a large U.S. national electronic medical records (EMR) database to identify 2LT accordingly: 1) retreatment after a treatment gap of >3 months of 1LT, or 2) a switch to another drug combination after starting 1LT. 3LT began with a switch to another regimen after 2LT. Pts with salvage stem cell transplants were excluded. Kaplan-Meier analyses were performed to calculate DOT from start of both 2LT and 3LT. Observations were censored at time of loss to follow up/end of study period (6/30/2015). A two-tailed log-rank test was used to test for statistical significance between groups.
Results
Among 628 pts, 37.1% were ≥75 years of age; 51.0% were male; 66.4% had ≥1 of the following comorbidities of interest at initiation of 2LT: diabetes, renal insufficiency, thromboembolic disease, cardiovascular disease, peripheral neuropathy. In 2LT, pts were most commonly treated with lenalidomide (L)-based regimens (36.2%) without bortezomib (B), followed by B-based therapies (33.0%, with/without L). Carfilzomib (C)-based treatments were least common (8.3%, with/without L). The remaining regimens (Other) comprised of MM-therapies without L, B, or C (22.6%). Overall, the median DOT in 2LT and 3LT was 6.9 months (mos) (95% CI: 5.9, 7.7) and 5.5 mos (95% CI: 4.0, 6.2), respectively. DOT in 2LT was similar for those <75 vs ≥75 years, median: 6.3 mos (95% CI: 5.2, 7.6) and 7.4 mos (95% CI: 5.9, 9.2), P>0.05, respectively (Table). Pts with baseline comorbidities of interest were significantly more likely to have a shorter DOT in 2LT, median: 6.2 mos (95% CI: 5.0, 7.4) compared to pts without, median: 9.2 mos (95% CI: 7.0, 11.9), P=0.02. In 2LT, the drug composition of the regimen was significantly associated with DOT (P<0.0001). L-based therapies had the longest DOT (median: 10.1 mos; 95% CI: 7.9, 11.9) compared to B-based (median: 6.6 mos; 95% CI: 5.1, 8.0), C-based (median: 4.6 mos; 95% CI: 3.0, 7.5) and Other regimens (median: 4.8 mos; 95% CI: 3.9, 6.2) (Figure).
Conclusion
DOT wanes with subsequent lines of therapy. Baseline comorbidities and regimen type in 2LT are significantly associated with DOT. The longest median DOT was observed with L-based therapies and the shortest DOT with C-based therapies. Tailored therapy choices that account for pts’ baseline comorbidities may mitigate the observed variation in DOT.References: Palumbo et al. JCO 2015;33:3459-66.Mateos et al. Am J Hematol.2015;90:314-9.

Session topic: E-poster
Keyword(s): Myeloma, Refractory, Relapse, Treatment
Type: Eposter Presentation
Background
Extended duration of therapy (DOT) is associated with better clinical outcomes in clinical trials in patients (pts) with multiple myeloma (MM) (Palumbo 2015; Mateos 2015). Many of the factors that influence DOT are more prevalent outside of clinical trials, including older age, and concomitant comorbidities. Real-world data related to DOT among RRMM pts are limited.
Aims
This study aims to 1) describe DOT of second line (2LT) and third line (3LT) therapy in RRMM, and 2) evaluate the associations between 2LT DOT and age; comorbidities; and regimen type.
Methods
In this retrospective cohort study, newly diagnosed adult MM pts initiating first-line therapy (1LT) between 1/2008 and 12/2014 were followed in a large U.S. national electronic medical records (EMR) database to identify 2LT accordingly: 1) retreatment after a treatment gap of >3 months of 1LT, or 2) a switch to another drug combination after starting 1LT. 3LT began with a switch to another regimen after 2LT. Pts with salvage stem cell transplants were excluded. Kaplan-Meier analyses were performed to calculate DOT from start of both 2LT and 3LT. Observations were censored at time of loss to follow up/end of study period (6/30/2015). A two-tailed log-rank test was used to test for statistical significance between groups.
Results
Among 628 pts, 37.1% were ≥75 years of age; 51.0% were male; 66.4% had ≥1 of the following comorbidities of interest at initiation of 2LT: diabetes, renal insufficiency, thromboembolic disease, cardiovascular disease, peripheral neuropathy. In 2LT, pts were most commonly treated with lenalidomide (L)-based regimens (36.2%) without bortezomib (B), followed by B-based therapies (33.0%, with/without L). Carfilzomib (C)-based treatments were least common (8.3%, with/without L). The remaining regimens (Other) comprised of MM-therapies without L, B, or C (22.6%). Overall, the median DOT in 2LT and 3LT was 6.9 months (mos) (95% CI: 5.9, 7.7) and 5.5 mos (95% CI: 4.0, 6.2), respectively. DOT in 2LT was similar for those <75 vs ≥75 years, median: 6.3 mos (95% CI: 5.2, 7.6) and 7.4 mos (95% CI: 5.9, 9.2), P>0.05, respectively (Table). Pts with baseline comorbidities of interest were significantly more likely to have a shorter DOT in 2LT, median: 6.2 mos (95% CI: 5.0, 7.4) compared to pts without, median: 9.2 mos (95% CI: 7.0, 11.9), P=0.02. In 2LT, the drug composition of the regimen was significantly associated with DOT (P<0.0001). L-based therapies had the longest DOT (median: 10.1 mos; 95% CI: 7.9, 11.9) compared to B-based (median: 6.6 mos; 95% CI: 5.1, 8.0), C-based (median: 4.6 mos; 95% CI: 3.0, 7.5) and Other regimens (median: 4.8 mos; 95% CI: 3.9, 6.2) (Figure).
Conclusion
DOT wanes with subsequent lines of therapy. Baseline comorbidities and regimen type in 2LT are significantly associated with DOT. The longest median DOT was observed with L-based therapies and the shortest DOT with C-based therapies. Tailored therapy choices that account for pts’ baseline comorbidities may mitigate the observed variation in DOT.References: Palumbo et al. JCO 2015;33:3459-66.Mateos et al. Am J Hematol.2015;90:314-9.

Session topic: E-poster
Keyword(s): Myeloma, Refractory, Relapse, Treatment
{{ help_message }}
{{filter}}


