OVERALL SURVIVAL IN PATIENTS WITH SYMPTOMATIC MULTIPLE MYELOMA IN THE REAL-WORLD SETTING: A RETROSPECTIVE ANALYSIS OF THE PHAROS REGISTRY IN THE NETHERLANDS
(Abstract release date: 05/19/16)
EHA Library. Verelst S. 06/09/16; 132841; E1292

Ms. Silvia Verelst
Contributions
Contributions
Abstract
Abstract: E1292
Type: Eposter Presentation
Background
Survival rates have improved for patients with multiple myeloma (MM), yet relapse remains common. Data on real-world outcomes in these patients in Europe is limited.
Aims
To provide insights into the survival of patients with symptomatic MM in the Netherlands through a retrospective analysis of data from the PHAROS registry, a Dutch population-based database.
Methods
The PHAROS registry included patients with MM diagnosed between 2004 and 2012 (aged ≥18 years). The study population consisted of 1522 patients starting first-, second- or third-line therapy in 2008 or later (not all patients in the analysis on second- and third-line therapy were included in the analysis of previous lines). The primary endpoint was overall survival (OS); secondary endpoints included progression-free survival (PFS) and healthcare resource utilisation (HRU).
Results
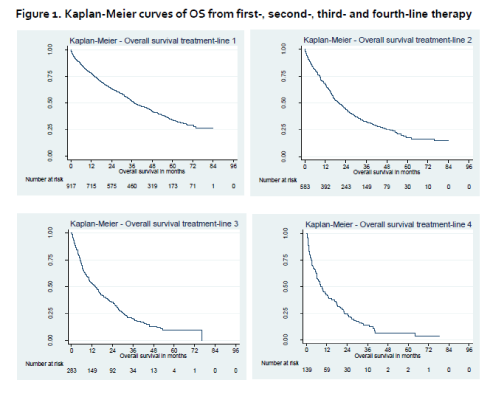
Median patient age was 70, 71, 71 and 72 at the initiation of first-, second-, third- and fourth-line therapies, respectively. Median follow-up for the primary endpoint (OS from first-line therapy) was 62.4 months (95% confidence interval [CI]: 60.6, 64.3). First-line thalidomide-based regimens were prescribed to 66% of patients (n=608) and bortezomib-based regimens to 15% (n=139). In the second-line setting (n=583), the majority of patients received bortezomib- (41%, n=239) or lenalidomide- (27%, n=159) based regimens. Median OS (95% confidence interval [CI]) in first- (n=917), second- (n=583) and third-line (n=283) were 37.5 (34.8–41.8), 19.7 (17.2–22.9) and 13.9 (10.5–16.6) months, respectively. Median PFS (95% CI) were 18.0 (16.3–18.9), 8.9 (7.9–9.7) and 6.4 (5.5–7.2) months, respectively. Large differences were seen between subgroups in all treatment-lines, e.g. median OS from first-line therapy was 31.9 (29.1-35.4) and 64.6 (53.2-not reached) months for patients >65 years old and patients ≤65 years old, respectively, and was 32.2 (29.2-35.5) for patients without prior stem cell transplantation (SCT) (median OS not reached for patients with prior SCT). Similarly, median PFS from first-line therapy was 16.2 (14.5-17.9) and 22.6 (19.8-26.5) months for patients >65 years old and patients ≤65 years old, respectively and 15.2 (13.6-17.0) and 32.0 (26.2-36.5) months for patients without or with prior SCT, respectively. Mean (standard deviation [SD]) number of inpatient days per month (excluding intensive care unit) were 1.7 (3.4), 1.4 (3.2) and 2.3 (4.6) in first, second and third-line, respectively.
Conclusion
In patients receiving first-line therapy for MM between 2008 and 2012 in The Netherlands, median OS was approximately three years. A difference in OS was observed for SCT versus non-SCT patients and resource use (inpatient stays) increased with later lines. Large differences in OS were also observed depending on a patient’s age.
Session topic: E-poster
Keyword(s): Multiple myeloma
Type: Eposter Presentation
Background
Survival rates have improved for patients with multiple myeloma (MM), yet relapse remains common. Data on real-world outcomes in these patients in Europe is limited.
Aims
To provide insights into the survival of patients with symptomatic MM in the Netherlands through a retrospective analysis of data from the PHAROS registry, a Dutch population-based database.
Methods
The PHAROS registry included patients with MM diagnosed between 2004 and 2012 (aged ≥18 years). The study population consisted of 1522 patients starting first-, second- or third-line therapy in 2008 or later (not all patients in the analysis on second- and third-line therapy were included in the analysis of previous lines). The primary endpoint was overall survival (OS); secondary endpoints included progression-free survival (PFS) and healthcare resource utilisation (HRU).
Results
Median patient age was 70, 71, 71 and 72 at the initiation of first-, second-, third- and fourth-line therapies, respectively. Median follow-up for the primary endpoint (OS from first-line therapy) was 62.4 months (95% confidence interval [CI]: 60.6, 64.3). First-line thalidomide-based regimens were prescribed to 66% of patients (n=608) and bortezomib-based regimens to 15% (n=139). In the second-line setting (n=583), the majority of patients received bortezomib- (41%, n=239) or lenalidomide- (27%, n=159) based regimens. Median OS (95% confidence interval [CI]) in first- (n=917), second- (n=583) and third-line (n=283) were 37.5 (34.8–41.8), 19.7 (17.2–22.9) and 13.9 (10.5–16.6) months, respectively. Median PFS (95% CI) were 18.0 (16.3–18.9), 8.9 (7.9–9.7) and 6.4 (5.5–7.2) months, respectively. Large differences were seen between subgroups in all treatment-lines, e.g. median OS from first-line therapy was 31.9 (29.1-35.4) and 64.6 (53.2-not reached) months for patients >65 years old and patients ≤65 years old, respectively, and was 32.2 (29.2-35.5) for patients without prior stem cell transplantation (SCT) (median OS not reached for patients with prior SCT). Similarly, median PFS from first-line therapy was 16.2 (14.5-17.9) and 22.6 (19.8-26.5) months for patients >65 years old and patients ≤65 years old, respectively and 15.2 (13.6-17.0) and 32.0 (26.2-36.5) months for patients without or with prior SCT, respectively. Mean (standard deviation [SD]) number of inpatient days per month (excluding intensive care unit) were 1.7 (3.4), 1.4 (3.2) and 2.3 (4.6) in first, second and third-line, respectively.
Conclusion
In patients receiving first-line therapy for MM between 2008 and 2012 in The Netherlands, median OS was approximately three years. A difference in OS was observed for SCT versus non-SCT patients and resource use (inpatient stays) increased with later lines. Large differences in OS were also observed depending on a patient’s age.

Session topic: E-poster
Keyword(s): Multiple myeloma
Abstract: E1292
Type: Eposter Presentation
Background
Survival rates have improved for patients with multiple myeloma (MM), yet relapse remains common. Data on real-world outcomes in these patients in Europe is limited.
Aims
To provide insights into the survival of patients with symptomatic MM in the Netherlands through a retrospective analysis of data from the PHAROS registry, a Dutch population-based database.
Methods
The PHAROS registry included patients with MM diagnosed between 2004 and 2012 (aged ≥18 years). The study population consisted of 1522 patients starting first-, second- or third-line therapy in 2008 or later (not all patients in the analysis on second- and third-line therapy were included in the analysis of previous lines). The primary endpoint was overall survival (OS); secondary endpoints included progression-free survival (PFS) and healthcare resource utilisation (HRU).
Results
Median patient age was 70, 71, 71 and 72 at the initiation of first-, second-, third- and fourth-line therapies, respectively. Median follow-up for the primary endpoint (OS from first-line therapy) was 62.4 months (95% confidence interval [CI]: 60.6, 64.3). First-line thalidomide-based regimens were prescribed to 66% of patients (n=608) and bortezomib-based regimens to 15% (n=139). In the second-line setting (n=583), the majority of patients received bortezomib- (41%, n=239) or lenalidomide- (27%, n=159) based regimens. Median OS (95% confidence interval [CI]) in first- (n=917), second- (n=583) and third-line (n=283) were 37.5 (34.8–41.8), 19.7 (17.2–22.9) and 13.9 (10.5–16.6) months, respectively. Median PFS (95% CI) were 18.0 (16.3–18.9), 8.9 (7.9–9.7) and 6.4 (5.5–7.2) months, respectively. Large differences were seen between subgroups in all treatment-lines, e.g. median OS from first-line therapy was 31.9 (29.1-35.4) and 64.6 (53.2-not reached) months for patients >65 years old and patients ≤65 years old, respectively, and was 32.2 (29.2-35.5) for patients without prior stem cell transplantation (SCT) (median OS not reached for patients with prior SCT). Similarly, median PFS from first-line therapy was 16.2 (14.5-17.9) and 22.6 (19.8-26.5) months for patients >65 years old and patients ≤65 years old, respectively and 15.2 (13.6-17.0) and 32.0 (26.2-36.5) months for patients without or with prior SCT, respectively. Mean (standard deviation [SD]) number of inpatient days per month (excluding intensive care unit) were 1.7 (3.4), 1.4 (3.2) and 2.3 (4.6) in first, second and third-line, respectively.
Conclusion
In patients receiving first-line therapy for MM between 2008 and 2012 in The Netherlands, median OS was approximately three years. A difference in OS was observed for SCT versus non-SCT patients and resource use (inpatient stays) increased with later lines. Large differences in OS were also observed depending on a patient’s age.

Session topic: E-poster
Keyword(s): Multiple myeloma
Type: Eposter Presentation
Background
Survival rates have improved for patients with multiple myeloma (MM), yet relapse remains common. Data on real-world outcomes in these patients in Europe is limited.
Aims
To provide insights into the survival of patients with symptomatic MM in the Netherlands through a retrospective analysis of data from the PHAROS registry, a Dutch population-based database.
Methods
The PHAROS registry included patients with MM diagnosed between 2004 and 2012 (aged ≥18 years). The study population consisted of 1522 patients starting first-, second- or third-line therapy in 2008 or later (not all patients in the analysis on second- and third-line therapy were included in the analysis of previous lines). The primary endpoint was overall survival (OS); secondary endpoints included progression-free survival (PFS) and healthcare resource utilisation (HRU).
Results
Median patient age was 70, 71, 71 and 72 at the initiation of first-, second-, third- and fourth-line therapies, respectively. Median follow-up for the primary endpoint (OS from first-line therapy) was 62.4 months (95% confidence interval [CI]: 60.6, 64.3). First-line thalidomide-based regimens were prescribed to 66% of patients (n=608) and bortezomib-based regimens to 15% (n=139). In the second-line setting (n=583), the majority of patients received bortezomib- (41%, n=239) or lenalidomide- (27%, n=159) based regimens. Median OS (95% confidence interval [CI]) in first- (n=917), second- (n=583) and third-line (n=283) were 37.5 (34.8–41.8), 19.7 (17.2–22.9) and 13.9 (10.5–16.6) months, respectively. Median PFS (95% CI) were 18.0 (16.3–18.9), 8.9 (7.9–9.7) and 6.4 (5.5–7.2) months, respectively. Large differences were seen between subgroups in all treatment-lines, e.g. median OS from first-line therapy was 31.9 (29.1-35.4) and 64.6 (53.2-not reached) months for patients >65 years old and patients ≤65 years old, respectively, and was 32.2 (29.2-35.5) for patients without prior stem cell transplantation (SCT) (median OS not reached for patients with prior SCT). Similarly, median PFS from first-line therapy was 16.2 (14.5-17.9) and 22.6 (19.8-26.5) months for patients >65 years old and patients ≤65 years old, respectively and 15.2 (13.6-17.0) and 32.0 (26.2-36.5) months for patients without or with prior SCT, respectively. Mean (standard deviation [SD]) number of inpatient days per month (excluding intensive care unit) were 1.7 (3.4), 1.4 (3.2) and 2.3 (4.6) in first, second and third-line, respectively.
Conclusion
In patients receiving first-line therapy for MM between 2008 and 2012 in The Netherlands, median OS was approximately three years. A difference in OS was observed for SCT versus non-SCT patients and resource use (inpatient stays) increased with later lines. Large differences in OS were also observed depending on a patient’s age.

Session topic: E-poster
Keyword(s): Multiple myeloma
{{ help_message }}
{{filter}}


