THE CLINICAL COURSE OF RELAPSED OR REFRACTORY U.S. MULTIPLE MYELOMA (RRMM) PATIENTS RECEIVING TWO OR MORE LINES OF THERAPY
(Abstract release date: 05/19/16)
EHA Library. Romanus D. 06/09/16; 132836; E1287

Dr. Dorothy Romanus
Contributions
Contributions
Abstract
Abstract: E1287
Type: Eposter Presentation
Background
The introduction of novel agents, proteasome inhibitors (PIs) and immunomodulatory drugs (IMIDs), has led to an improvement in prognosis in multiple myeloma (MM). However, over the course of the disease, less durable remissions with each successive therapy have been noted in MM. In a retrospective single-institution, chart review study, patients experienced shorter overall survival (OS) as they progressed through each line of therapy, first-line (1LT) and second-line (2LT), OS of 28.4 and 17.1 months, respectively (Kumar 2004). Other evidence from a multi-center observational analysis of 383 MM patients (treated between 2007 and 2010) reported a median progression free (PFS) and OS from start of salvage therapy after first relapse of 13 and 35 months, respectively (Durie 2012); however, the clinical course of RRMM in the era of novel agents among pts treated in the real-world remains to be elucidated.
Aims
This study aims to describe the PFS and OS of RRMM patients receiving two or more lines of therapy in the era of novel agents in a large national database in the U.S.
Methods
In this retrospective cohort study using an electronic medical records (EMR) database from 38 states in the U.S, newly diagnosed, adult MM patients initiating 1LT between 1/2008 and 12/2014 were followed to identify 2LT accordingly: 1) retreatment after a treatment gap of >3 months of a prior LT, or 2) a switch to another drug combination after 1LT initiation. Subsequent lines of therapy, 3LT and 4LT, began with a switch in regimen compared to the previous line. Pts with salvage stem cell transplants (SCT) were excluded. Time to new treatment or death (TTNT) was used as a surrogate for PFS. Kaplan-Meier analyses were performed for OS/TTNT from start of each line of therapy. Observations were censored at time of loss to follow up or end of study period (6/30/2015).
Results
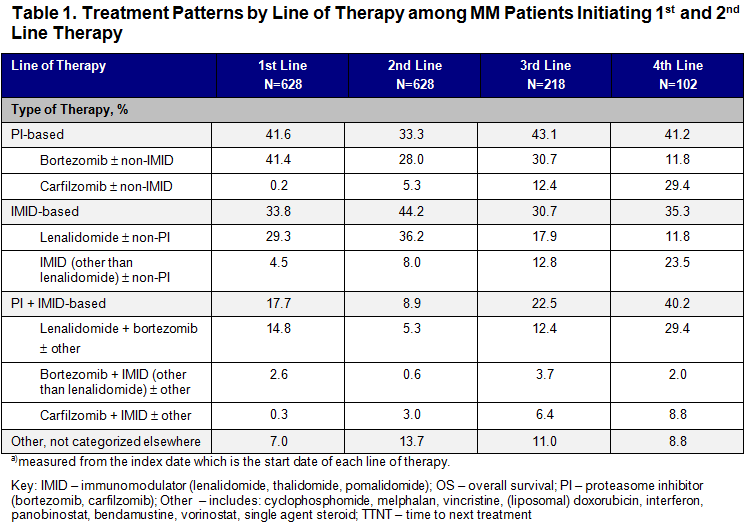
Among 628 patients initiating both 1LT and 2LT, mean age was 69.2 years (SD: 10.3) at start of 2LT; 51% were male; 9.6% had known high cytogenetic risk MM (del[17p] and/or t[4:14] and/or t[14:16]); 19.6% had a frontline SCT; 46.7% had a Charlson Comorbidity Index (CCI) score ≥2. PI-based regimens predominated in 1LT (44.2%), 3LT (43.1%) and in 4LT (41.2%) (Table 1). The most common regimen in 2LT was IMID-based (44.2%). PI + IMID-based combination therapy increased from 17.7% in 1LT to 40.2% in 4LT. As patients progressed through lines of therapy, median TTNT decreased from 15.1 months (95% CI: 13.4, 17.3) in 2LT to 7.8 months (95% CI: 6.1, 9.7) in 3LT and 6.9 months (95% CI: 5.1, 11.8) in 4LT. Median OS decreased from 41.0 months (95% CI: 32.1, 59.5) in 2LT to 30.3 months (95% CI: 20.1, 46.0) in 3LT and 24.8 months (95% CI: 12.8, 43.0) in 4LT.
Conclusion
Median OS and time to progression from 2LT was longer than previously reported (Durie 2012; Kumar 2004). However, successive treatment regimens yielded progressively shorter TTNT and OS durations. These data may support treatment decision making by informing the trade-offs between prognosis, treatment effectiveness and toxicity.References:Durie ASCO 2012; abs 8095 Kumar, et al. Mayo Clin Proc. 2004; 79(7):867-874
Session topic: E-poster
Keyword(s): Myeloma, Relapse, Survival
Type: Eposter Presentation
Background
The introduction of novel agents, proteasome inhibitors (PIs) and immunomodulatory drugs (IMIDs), has led to an improvement in prognosis in multiple myeloma (MM). However, over the course of the disease, less durable remissions with each successive therapy have been noted in MM. In a retrospective single-institution, chart review study, patients experienced shorter overall survival (OS) as they progressed through each line of therapy, first-line (1LT) and second-line (2LT), OS of 28.4 and 17.1 months, respectively (Kumar 2004). Other evidence from a multi-center observational analysis of 383 MM patients (treated between 2007 and 2010) reported a median progression free (PFS) and OS from start of salvage therapy after first relapse of 13 and 35 months, respectively (Durie 2012); however, the clinical course of RRMM in the era of novel agents among pts treated in the real-world remains to be elucidated.
Aims
This study aims to describe the PFS and OS of RRMM patients receiving two or more lines of therapy in the era of novel agents in a large national database in the U.S.
Methods
In this retrospective cohort study using an electronic medical records (EMR) database from 38 states in the U.S, newly diagnosed, adult MM patients initiating 1LT between 1/2008 and 12/2014 were followed to identify 2LT accordingly: 1) retreatment after a treatment gap of >3 months of a prior LT, or 2) a switch to another drug combination after 1LT initiation. Subsequent lines of therapy, 3LT and 4LT, began with a switch in regimen compared to the previous line. Pts with salvage stem cell transplants (SCT) were excluded. Time to new treatment or death (TTNT) was used as a surrogate for PFS. Kaplan-Meier analyses were performed for OS/TTNT from start of each line of therapy. Observations were censored at time of loss to follow up or end of study period (6/30/2015).
Results
Among 628 patients initiating both 1LT and 2LT, mean age was 69.2 years (SD: 10.3) at start of 2LT; 51% were male; 9.6% had known high cytogenetic risk MM (del[17p] and/or t[4:14] and/or t[14:16]); 19.6% had a frontline SCT; 46.7% had a Charlson Comorbidity Index (CCI) score ≥2. PI-based regimens predominated in 1LT (44.2%), 3LT (43.1%) and in 4LT (41.2%) (Table 1). The most common regimen in 2LT was IMID-based (44.2%). PI + IMID-based combination therapy increased from 17.7% in 1LT to 40.2% in 4LT. As patients progressed through lines of therapy, median TTNT decreased from 15.1 months (95% CI: 13.4, 17.3) in 2LT to 7.8 months (95% CI: 6.1, 9.7) in 3LT and 6.9 months (95% CI: 5.1, 11.8) in 4LT. Median OS decreased from 41.0 months (95% CI: 32.1, 59.5) in 2LT to 30.3 months (95% CI: 20.1, 46.0) in 3LT and 24.8 months (95% CI: 12.8, 43.0) in 4LT.
Conclusion
Median OS and time to progression from 2LT was longer than previously reported (Durie 2012; Kumar 2004). However, successive treatment regimens yielded progressively shorter TTNT and OS durations. These data may support treatment decision making by informing the trade-offs between prognosis, treatment effectiveness and toxicity.References:Durie ASCO 2012; abs 8095 Kumar, et al. Mayo Clin Proc. 2004; 79(7):867-874

Session topic: E-poster
Keyword(s): Myeloma, Relapse, Survival
Abstract: E1287
Type: Eposter Presentation
Background
The introduction of novel agents, proteasome inhibitors (PIs) and immunomodulatory drugs (IMIDs), has led to an improvement in prognosis in multiple myeloma (MM). However, over the course of the disease, less durable remissions with each successive therapy have been noted in MM. In a retrospective single-institution, chart review study, patients experienced shorter overall survival (OS) as they progressed through each line of therapy, first-line (1LT) and second-line (2LT), OS of 28.4 and 17.1 months, respectively (Kumar 2004). Other evidence from a multi-center observational analysis of 383 MM patients (treated between 2007 and 2010) reported a median progression free (PFS) and OS from start of salvage therapy after first relapse of 13 and 35 months, respectively (Durie 2012); however, the clinical course of RRMM in the era of novel agents among pts treated in the real-world remains to be elucidated.
Aims
This study aims to describe the PFS and OS of RRMM patients receiving two or more lines of therapy in the era of novel agents in a large national database in the U.S.
Methods
In this retrospective cohort study using an electronic medical records (EMR) database from 38 states in the U.S, newly diagnosed, adult MM patients initiating 1LT between 1/2008 and 12/2014 were followed to identify 2LT accordingly: 1) retreatment after a treatment gap of >3 months of a prior LT, or 2) a switch to another drug combination after 1LT initiation. Subsequent lines of therapy, 3LT and 4LT, began with a switch in regimen compared to the previous line. Pts with salvage stem cell transplants (SCT) were excluded. Time to new treatment or death (TTNT) was used as a surrogate for PFS. Kaplan-Meier analyses were performed for OS/TTNT from start of each line of therapy. Observations were censored at time of loss to follow up or end of study period (6/30/2015).
Results
Among 628 patients initiating both 1LT and 2LT, mean age was 69.2 years (SD: 10.3) at start of 2LT; 51% were male; 9.6% had known high cytogenetic risk MM (del[17p] and/or t[4:14] and/or t[14:16]); 19.6% had a frontline SCT; 46.7% had a Charlson Comorbidity Index (CCI) score ≥2. PI-based regimens predominated in 1LT (44.2%), 3LT (43.1%) and in 4LT (41.2%) (Table 1). The most common regimen in 2LT was IMID-based (44.2%). PI + IMID-based combination therapy increased from 17.7% in 1LT to 40.2% in 4LT. As patients progressed through lines of therapy, median TTNT decreased from 15.1 months (95% CI: 13.4, 17.3) in 2LT to 7.8 months (95% CI: 6.1, 9.7) in 3LT and 6.9 months (95% CI: 5.1, 11.8) in 4LT. Median OS decreased from 41.0 months (95% CI: 32.1, 59.5) in 2LT to 30.3 months (95% CI: 20.1, 46.0) in 3LT and 24.8 months (95% CI: 12.8, 43.0) in 4LT.
Conclusion
Median OS and time to progression from 2LT was longer than previously reported (Durie 2012; Kumar 2004). However, successive treatment regimens yielded progressively shorter TTNT and OS durations. These data may support treatment decision making by informing the trade-offs between prognosis, treatment effectiveness and toxicity.References:Durie ASCO 2012; abs 8095 Kumar, et al. Mayo Clin Proc. 2004; 79(7):867-874

Session topic: E-poster
Keyword(s): Myeloma, Relapse, Survival
Type: Eposter Presentation
Background
The introduction of novel agents, proteasome inhibitors (PIs) and immunomodulatory drugs (IMIDs), has led to an improvement in prognosis in multiple myeloma (MM). However, over the course of the disease, less durable remissions with each successive therapy have been noted in MM. In a retrospective single-institution, chart review study, patients experienced shorter overall survival (OS) as they progressed through each line of therapy, first-line (1LT) and second-line (2LT), OS of 28.4 and 17.1 months, respectively (Kumar 2004). Other evidence from a multi-center observational analysis of 383 MM patients (treated between 2007 and 2010) reported a median progression free (PFS) and OS from start of salvage therapy after first relapse of 13 and 35 months, respectively (Durie 2012); however, the clinical course of RRMM in the era of novel agents among pts treated in the real-world remains to be elucidated.
Aims
This study aims to describe the PFS and OS of RRMM patients receiving two or more lines of therapy in the era of novel agents in a large national database in the U.S.
Methods
In this retrospective cohort study using an electronic medical records (EMR) database from 38 states in the U.S, newly diagnosed, adult MM patients initiating 1LT between 1/2008 and 12/2014 were followed to identify 2LT accordingly: 1) retreatment after a treatment gap of >3 months of a prior LT, or 2) a switch to another drug combination after 1LT initiation. Subsequent lines of therapy, 3LT and 4LT, began with a switch in regimen compared to the previous line. Pts with salvage stem cell transplants (SCT) were excluded. Time to new treatment or death (TTNT) was used as a surrogate for PFS. Kaplan-Meier analyses were performed for OS/TTNT from start of each line of therapy. Observations were censored at time of loss to follow up or end of study period (6/30/2015).
Results
Among 628 patients initiating both 1LT and 2LT, mean age was 69.2 years (SD: 10.3) at start of 2LT; 51% were male; 9.6% had known high cytogenetic risk MM (del[17p] and/or t[4:14] and/or t[14:16]); 19.6% had a frontline SCT; 46.7% had a Charlson Comorbidity Index (CCI) score ≥2. PI-based regimens predominated in 1LT (44.2%), 3LT (43.1%) and in 4LT (41.2%) (Table 1). The most common regimen in 2LT was IMID-based (44.2%). PI + IMID-based combination therapy increased from 17.7% in 1LT to 40.2% in 4LT. As patients progressed through lines of therapy, median TTNT decreased from 15.1 months (95% CI: 13.4, 17.3) in 2LT to 7.8 months (95% CI: 6.1, 9.7) in 3LT and 6.9 months (95% CI: 5.1, 11.8) in 4LT. Median OS decreased from 41.0 months (95% CI: 32.1, 59.5) in 2LT to 30.3 months (95% CI: 20.1, 46.0) in 3LT and 24.8 months (95% CI: 12.8, 43.0) in 4LT.
Conclusion
Median OS and time to progression from 2LT was longer than previously reported (Durie 2012; Kumar 2004). However, successive treatment regimens yielded progressively shorter TTNT and OS durations. These data may support treatment decision making by informing the trade-offs between prognosis, treatment effectiveness and toxicity.References:Durie ASCO 2012; abs 8095 Kumar, et al. Mayo Clin Proc. 2004; 79(7):867-874

Session topic: E-poster
Keyword(s): Myeloma, Relapse, Survival
{{ help_message }}
{{filter}}


