AN ONGOING MULTINATIONAL OBSERVATIONAL STUDY IN MULTIPLE MYELOMA (PREAMBLE): PRELIMINARY REPORT ON PROGRESSION-FREE SURVIVAL
(Abstract release date: 05/19/16)
EHA Library. Zyczynski T. 06/09/16; 132810; E1261

Teresa Zyczynski
Contributions
Contributions
Abstract
Abstract: E1261
Type: Eposter Presentation
Background
Multiple myeloma (MM) is associated with significant disease burden, and long-term prognosis is poor, with relapse almost inevitable. Immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs) have improved patient outcomes; however, data on real-world clinical effectiveness of these available treatments are limited.
Aims
PREAMBLE (Prospective REsearch Assessment in multiple Myeloma: an oBservationaL Evaluation; NCT01838512) is an ongoing, international, prospective, observational cohort study to improve understanding of the real-world outcomes of IMiDs, PIs, and combination therapy in relapsed/refractory MM (RRMM). Here we report preliminary progression-free survival (PFS) data.
Methods
Patients with RRMM (≥1 prior therapy) who initiated treatment with an IMiD, PI, or IMiD+PI within 90 days before or 30 days after study enrollment were eligible. Data were analyzed using a Cox proportional hazard regression model that included patient demographics, baseline disease characteristics, and MM treatment-related variables as covariates.
Results
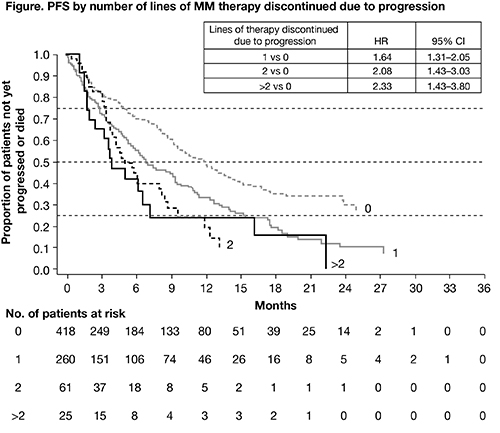
At the time of data cut-off (December 7, 2015), data were available for 764 patients and median duration of follow-up was 15 months. The median age of patients was 68 years and 55% were male. Of the 764 patients, 368 (48%) were receiving an IMiD (81% of whom were receiving lenalidomide, n=297), 347 (45%) were receiving a PI (80% of whom were receiving bortezomib, n=278), and 49 (6%) were receiving an IMiD+PI (59% of whom were receiving lenalidomide/bortezomib, n=29). Most patients (n=325; 43%) had 1 prior line of therapy, 203 (27%) had 2 prior lines, 111 (15%) had 3 prior lines, and 123 (16%) had >3. The majority of patients had relapsed MM (n=599, 78%). 161 (21%) patients were refractory to their last line of treatment; 260 (34%) patients discontinued 1 prior line of therapy due to progression, 61 (8%) discontinued 2 prior lines due to progression, and 25 (3%) discontinued >2 prior lines. 40% of patients had International Staging System (ISS) stage III disease. Median PFS was 9.26 months (95% CI 8.21–10.12); 1-year PFS rate was 40.9% and 2-year PFS rate was 19.7%. Approximately half of the patients (50.7%) were estimated to respond to treatment before then progressing. At 2 years, all patients in this analysis had either responded to treatment or progressed without response. Median (95% CI) duration of response was 14.2 (10.87–16.39) months and was longer for patients receiving an IMiD versus a PI (16.4 [11.73–not estimable] months vs 10.9 [7.13–14.69] months). Number of lines of therapy discontinued due to progression was most strongly associated with higher rates of progression/death (Figure; HR p<0.001 for all comparisons). Other factors associated with higher rates of progression or death were number of prior lines of therapy (2 vs 1, HR 1.38, p=0.016; 3 vs 1, HR 1.48, p=0.012; >3 vs 1, HR 1.63, p<0.001) and ISS stage at study entry (III vs I, HR 1.45, p=0.032).
Conclusion
Preliminary analysis of real-world data from PREAMBLE suggests that the number of lines of therapy discontinued due to progression, lines of therapy overall, and disease stage are key risk factors associated with the rate of progression/death in patients with MM, demonstrating an unmet need.Funding: Bristol-Myers Squibb. Medical writing assistance provided by A Bexfield, Caudex, funded by Bristol-Myers Squibb.
Session topic: E-poster
Keyword(s): Imids, Multiple myeloma, Proteasome inhibitor, Treatment-related mortality
Type: Eposter Presentation
Background
Multiple myeloma (MM) is associated with significant disease burden, and long-term prognosis is poor, with relapse almost inevitable. Immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs) have improved patient outcomes; however, data on real-world clinical effectiveness of these available treatments are limited.
Aims
PREAMBLE (Prospective REsearch Assessment in multiple Myeloma: an oBservationaL Evaluation; NCT01838512) is an ongoing, international, prospective, observational cohort study to improve understanding of the real-world outcomes of IMiDs, PIs, and combination therapy in relapsed/refractory MM (RRMM). Here we report preliminary progression-free survival (PFS) data.
Methods
Patients with RRMM (≥1 prior therapy) who initiated treatment with an IMiD, PI, or IMiD+PI within 90 days before or 30 days after study enrollment were eligible. Data were analyzed using a Cox proportional hazard regression model that included patient demographics, baseline disease characteristics, and MM treatment-related variables as covariates.
Results
At the time of data cut-off (December 7, 2015), data were available for 764 patients and median duration of follow-up was 15 months. The median age of patients was 68 years and 55% were male. Of the 764 patients, 368 (48%) were receiving an IMiD (81% of whom were receiving lenalidomide, n=297), 347 (45%) were receiving a PI (80% of whom were receiving bortezomib, n=278), and 49 (6%) were receiving an IMiD+PI (59% of whom were receiving lenalidomide/bortezomib, n=29). Most patients (n=325; 43%) had 1 prior line of therapy, 203 (27%) had 2 prior lines, 111 (15%) had 3 prior lines, and 123 (16%) had >3. The majority of patients had relapsed MM (n=599, 78%). 161 (21%) patients were refractory to their last line of treatment; 260 (34%) patients discontinued 1 prior line of therapy due to progression, 61 (8%) discontinued 2 prior lines due to progression, and 25 (3%) discontinued >2 prior lines. 40% of patients had International Staging System (ISS) stage III disease. Median PFS was 9.26 months (95% CI 8.21–10.12); 1-year PFS rate was 40.9% and 2-year PFS rate was 19.7%. Approximately half of the patients (50.7%) were estimated to respond to treatment before then progressing. At 2 years, all patients in this analysis had either responded to treatment or progressed without response. Median (95% CI) duration of response was 14.2 (10.87–16.39) months and was longer for patients receiving an IMiD versus a PI (16.4 [11.73–not estimable] months vs 10.9 [7.13–14.69] months). Number of lines of therapy discontinued due to progression was most strongly associated with higher rates of progression/death (Figure; HR p<0.001 for all comparisons). Other factors associated with higher rates of progression or death were number of prior lines of therapy (2 vs 1, HR 1.38, p=0.016; 3 vs 1, HR 1.48, p=0.012; >3 vs 1, HR 1.63, p<0.001) and ISS stage at study entry (III vs I, HR 1.45, p=0.032).
Conclusion
Preliminary analysis of real-world data from PREAMBLE suggests that the number of lines of therapy discontinued due to progression, lines of therapy overall, and disease stage are key risk factors associated with the rate of progression/death in patients with MM, demonstrating an unmet need.Funding: Bristol-Myers Squibb. Medical writing assistance provided by A Bexfield, Caudex, funded by Bristol-Myers Squibb.

Session topic: E-poster
Keyword(s): Imids, Multiple myeloma, Proteasome inhibitor, Treatment-related mortality
Abstract: E1261
Type: Eposter Presentation
Background
Multiple myeloma (MM) is associated with significant disease burden, and long-term prognosis is poor, with relapse almost inevitable. Immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs) have improved patient outcomes; however, data on real-world clinical effectiveness of these available treatments are limited.
Aims
PREAMBLE (Prospective REsearch Assessment in multiple Myeloma: an oBservationaL Evaluation; NCT01838512) is an ongoing, international, prospective, observational cohort study to improve understanding of the real-world outcomes of IMiDs, PIs, and combination therapy in relapsed/refractory MM (RRMM). Here we report preliminary progression-free survival (PFS) data.
Methods
Patients with RRMM (≥1 prior therapy) who initiated treatment with an IMiD, PI, or IMiD+PI within 90 days before or 30 days after study enrollment were eligible. Data were analyzed using a Cox proportional hazard regression model that included patient demographics, baseline disease characteristics, and MM treatment-related variables as covariates.
Results
At the time of data cut-off (December 7, 2015), data were available for 764 patients and median duration of follow-up was 15 months. The median age of patients was 68 years and 55% were male. Of the 764 patients, 368 (48%) were receiving an IMiD (81% of whom were receiving lenalidomide, n=297), 347 (45%) were receiving a PI (80% of whom were receiving bortezomib, n=278), and 49 (6%) were receiving an IMiD+PI (59% of whom were receiving lenalidomide/bortezomib, n=29). Most patients (n=325; 43%) had 1 prior line of therapy, 203 (27%) had 2 prior lines, 111 (15%) had 3 prior lines, and 123 (16%) had >3. The majority of patients had relapsed MM (n=599, 78%). 161 (21%) patients were refractory to their last line of treatment; 260 (34%) patients discontinued 1 prior line of therapy due to progression, 61 (8%) discontinued 2 prior lines due to progression, and 25 (3%) discontinued >2 prior lines. 40% of patients had International Staging System (ISS) stage III disease. Median PFS was 9.26 months (95% CI 8.21–10.12); 1-year PFS rate was 40.9% and 2-year PFS rate was 19.7%. Approximately half of the patients (50.7%) were estimated to respond to treatment before then progressing. At 2 years, all patients in this analysis had either responded to treatment or progressed without response. Median (95% CI) duration of response was 14.2 (10.87–16.39) months and was longer for patients receiving an IMiD versus a PI (16.4 [11.73–not estimable] months vs 10.9 [7.13–14.69] months). Number of lines of therapy discontinued due to progression was most strongly associated with higher rates of progression/death (Figure; HR p<0.001 for all comparisons). Other factors associated with higher rates of progression or death were number of prior lines of therapy (2 vs 1, HR 1.38, p=0.016; 3 vs 1, HR 1.48, p=0.012; >3 vs 1, HR 1.63, p<0.001) and ISS stage at study entry (III vs I, HR 1.45, p=0.032).
Conclusion
Preliminary analysis of real-world data from PREAMBLE suggests that the number of lines of therapy discontinued due to progression, lines of therapy overall, and disease stage are key risk factors associated with the rate of progression/death in patients with MM, demonstrating an unmet need.Funding: Bristol-Myers Squibb. Medical writing assistance provided by A Bexfield, Caudex, funded by Bristol-Myers Squibb.

Session topic: E-poster
Keyword(s): Imids, Multiple myeloma, Proteasome inhibitor, Treatment-related mortality
Type: Eposter Presentation
Background
Multiple myeloma (MM) is associated with significant disease burden, and long-term prognosis is poor, with relapse almost inevitable. Immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs) have improved patient outcomes; however, data on real-world clinical effectiveness of these available treatments are limited.
Aims
PREAMBLE (Prospective REsearch Assessment in multiple Myeloma: an oBservationaL Evaluation; NCT01838512) is an ongoing, international, prospective, observational cohort study to improve understanding of the real-world outcomes of IMiDs, PIs, and combination therapy in relapsed/refractory MM (RRMM). Here we report preliminary progression-free survival (PFS) data.
Methods
Patients with RRMM (≥1 prior therapy) who initiated treatment with an IMiD, PI, or IMiD+PI within 90 days before or 30 days after study enrollment were eligible. Data were analyzed using a Cox proportional hazard regression model that included patient demographics, baseline disease characteristics, and MM treatment-related variables as covariates.
Results
At the time of data cut-off (December 7, 2015), data were available for 764 patients and median duration of follow-up was 15 months. The median age of patients was 68 years and 55% were male. Of the 764 patients, 368 (48%) were receiving an IMiD (81% of whom were receiving lenalidomide, n=297), 347 (45%) were receiving a PI (80% of whom were receiving bortezomib, n=278), and 49 (6%) were receiving an IMiD+PI (59% of whom were receiving lenalidomide/bortezomib, n=29). Most patients (n=325; 43%) had 1 prior line of therapy, 203 (27%) had 2 prior lines, 111 (15%) had 3 prior lines, and 123 (16%) had >3. The majority of patients had relapsed MM (n=599, 78%). 161 (21%) patients were refractory to their last line of treatment; 260 (34%) patients discontinued 1 prior line of therapy due to progression, 61 (8%) discontinued 2 prior lines due to progression, and 25 (3%) discontinued >2 prior lines. 40% of patients had International Staging System (ISS) stage III disease. Median PFS was 9.26 months (95% CI 8.21–10.12); 1-year PFS rate was 40.9% and 2-year PFS rate was 19.7%. Approximately half of the patients (50.7%) were estimated to respond to treatment before then progressing. At 2 years, all patients in this analysis had either responded to treatment or progressed without response. Median (95% CI) duration of response was 14.2 (10.87–16.39) months and was longer for patients receiving an IMiD versus a PI (16.4 [11.73–not estimable] months vs 10.9 [7.13–14.69] months). Number of lines of therapy discontinued due to progression was most strongly associated with higher rates of progression/death (Figure; HR p<0.001 for all comparisons). Other factors associated with higher rates of progression or death were number of prior lines of therapy (2 vs 1, HR 1.38, p=0.016; 3 vs 1, HR 1.48, p=0.012; >3 vs 1, HR 1.63, p<0.001) and ISS stage at study entry (III vs I, HR 1.45, p=0.032).
Conclusion
Preliminary analysis of real-world data from PREAMBLE suggests that the number of lines of therapy discontinued due to progression, lines of therapy overall, and disease stage are key risk factors associated with the rate of progression/death in patients with MM, demonstrating an unmet need.Funding: Bristol-Myers Squibb. Medical writing assistance provided by A Bexfield, Caudex, funded by Bristol-Myers Squibb.

Session topic: E-poster
Keyword(s): Imids, Multiple myeloma, Proteasome inhibitor, Treatment-related mortality
{{ help_message }}
{{filter}}


