PATTERNS OF IDELALISIB TREATMENT-EMERGENT LYMPHOCYTOSIS IN PATIENTS WITH CLL OR SLL
(Abstract release date: 05/19/16)
EHA Library. Ghia P. 06/09/16; 132616; E1067

Dr. Paolo Ghia
Contributions
Contributions
Abstract
Abstract: E1067
Type: Eposter Presentation
Background
Lymphocytosis is a known effect of B-cell receptor (BCR) targeted therapies used to treat CLL and SLL, including idelalisib (IDELA). IDELA is a selective oral PI3Kδ inhibitor which inhibits multiple signaling pathways, including those downstream of the B-cell receptor, CXCR4, and CXCR5. Per updated response criteria (Hallek M, el al. Blood. 2012;119:5348; Cheson B, et al. J Clin Oncol. 2012;30:2820-2822.), drug-induced lymphocytosis alone should not interfere with the time of designation of a partial response (PR) and is not considered progressive disease (PD) in the absence of other signs or symptoms of progression.
Aims
This post hoc analysis characterized the patterns of lymphocytosis observed in patients (pts) with CLL/SLL treated with IDELA-based regimens, and evaluated the effect of lymphocytosis on safety and efficacy.
Methods
Pts from 4 studies (2 phase 1, 1 phase 2 and 1 phase 3) who received IDELA dosed at 100 or 150 mg BID were included in this analysis. Pts were grouped by disease status (treatment-naïve vs relapsed/refractory) and treatment regimen (IDELA-monotherapy vs IDELA in combination with chemotherapy, an anti-CD20 mAb [anti-CD20], or both. Chemotherapeutic agents included chlorambucil, bendamustine, or fludarabine. Anti-CD20 mAbs included rituximab or ofatumumab. Absolute lymphocyte counts (ALC) were measured throughout each study to calculate peak ALC, time to peak ALC, and time to 50% reduction from baseline ALC. To evaluate the effect of lymphocytosis on safety, grade ≥3 treatment-emergent adverse events (TEAEs) and TEAEs of interest (leukostasis, blood viscosity abnormalities, central nervous system hemorrhage, and disseminated intravascular coagulation) were summarized in pts with ALC >200 K/µl at any time during IDELA treatment. To evaluate the effect of lymphocytosis on efficacy, median progression-free survival (PFS) was analyzed in pts receiving IDELA monotherapy for relapsed/refractory CLL (phase 1 study) and treatment-naive CLL/SLL (phase 2 study) stratified by ALC reduction from baseline (≥50% [yes or no]).
Results
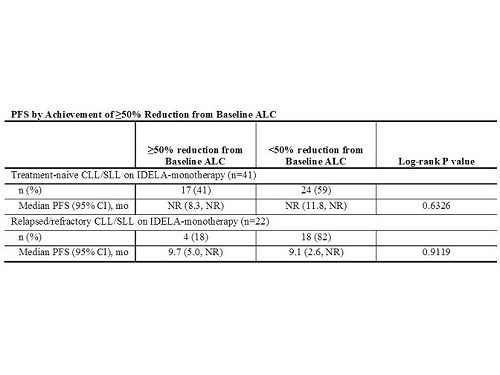
The analysis population included 352 pts (105 treatment-naïve; 247 relapsed/refractory), 63 on IDELA monotherapy and 289 on IDELA combination regimens. 68% of pts experienced a post-baseline increase in ALC, including 83% of pts on IDELA monotherapy and 50% of pts on combinations. Time to peak was 2-4 weeks across groups. 73% of pts achieved a 50% reduction from baseline ALC, including 33% of pts on IDELA monotherapy and 82% of pts on combinations. Median time to 50% reduction from baseline ALC was longest for pts with relapsed/refractory CLL/SLL on IDELA monotherapy (15 wks) and shortest for pts with relapsed/refractory CLL on IDELA+chemotherapy+anti-CD20 (2 wks). The safety analysis included 31/352 pts (9%) with ALC >200 K/µl at any time on IDELA treatment, with a maximum reported ALC of 447 K/µl. Of these 31 pts, Grade 3/4 TEAEs were reported in 8 (26%) during the time when ALC >200 K/µl. No pt with signs or symptoms of hyperleukocytosis was identified.The efficacy analysis included 63 pts (41 treatment-naïve; 22 relapsed/refractory) on IDELA-monotherapy. The median PFS was similar for pts with or without a ≥50% reduction from baseline ALC (Table).
Conclusion
Addition of anti-CD20 and/or chemotherapy to IDELA abrogated the transient lymphocytosis seen with IDELA-monotherapy, regardless of disease status. Consistent with the updated response criteria, lymphocytosis does not appear to impact the efficacy of IDELA in this pt population. Lymphocytosis also did not predispose pts to severe or unexpected AEs.
Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Lymphocyte, Progression, Targeted therapy
Type: Eposter Presentation
Background
Lymphocytosis is a known effect of B-cell receptor (BCR) targeted therapies used to treat CLL and SLL, including idelalisib (IDELA). IDELA is a selective oral PI3Kδ inhibitor which inhibits multiple signaling pathways, including those downstream of the B-cell receptor, CXCR4, and CXCR5. Per updated response criteria (Hallek M, el al. Blood. 2012;119:5348; Cheson B, et al. J Clin Oncol. 2012;30:2820-2822.), drug-induced lymphocytosis alone should not interfere with the time of designation of a partial response (PR) and is not considered progressive disease (PD) in the absence of other signs or symptoms of progression.
Aims
This post hoc analysis characterized the patterns of lymphocytosis observed in patients (pts) with CLL/SLL treated with IDELA-based regimens, and evaluated the effect of lymphocytosis on safety and efficacy.
Methods
Pts from 4 studies (2 phase 1, 1 phase 2 and 1 phase 3) who received IDELA dosed at 100 or 150 mg BID were included in this analysis. Pts were grouped by disease status (treatment-naïve vs relapsed/refractory) and treatment regimen (IDELA-monotherapy vs IDELA in combination with chemotherapy, an anti-CD20 mAb [anti-CD20], or both. Chemotherapeutic agents included chlorambucil, bendamustine, or fludarabine. Anti-CD20 mAbs included rituximab or ofatumumab. Absolute lymphocyte counts (ALC) were measured throughout each study to calculate peak ALC, time to peak ALC, and time to 50% reduction from baseline ALC. To evaluate the effect of lymphocytosis on safety, grade ≥3 treatment-emergent adverse events (TEAEs) and TEAEs of interest (leukostasis, blood viscosity abnormalities, central nervous system hemorrhage, and disseminated intravascular coagulation) were summarized in pts with ALC >200 K/µl at any time during IDELA treatment. To evaluate the effect of lymphocytosis on efficacy, median progression-free survival (PFS) was analyzed in pts receiving IDELA monotherapy for relapsed/refractory CLL (phase 1 study) and treatment-naive CLL/SLL (phase 2 study) stratified by ALC reduction from baseline (≥50% [yes or no]).
Results
The analysis population included 352 pts (105 treatment-naïve; 247 relapsed/refractory), 63 on IDELA monotherapy and 289 on IDELA combination regimens. 68% of pts experienced a post-baseline increase in ALC, including 83% of pts on IDELA monotherapy and 50% of pts on combinations. Time to peak was 2-4 weeks across groups. 73% of pts achieved a 50% reduction from baseline ALC, including 33% of pts on IDELA monotherapy and 82% of pts on combinations. Median time to 50% reduction from baseline ALC was longest for pts with relapsed/refractory CLL/SLL on IDELA monotherapy (15 wks) and shortest for pts with relapsed/refractory CLL on IDELA+chemotherapy+anti-CD20 (2 wks). The safety analysis included 31/352 pts (9%) with ALC >200 K/µl at any time on IDELA treatment, with a maximum reported ALC of 447 K/µl. Of these 31 pts, Grade 3/4 TEAEs were reported in 8 (26%) during the time when ALC >200 K/µl. No pt with signs or symptoms of hyperleukocytosis was identified.The efficacy analysis included 63 pts (41 treatment-naïve; 22 relapsed/refractory) on IDELA-monotherapy. The median PFS was similar for pts with or without a ≥50% reduction from baseline ALC (Table).
Conclusion
Addition of anti-CD20 and/or chemotherapy to IDELA abrogated the transient lymphocytosis seen with IDELA-monotherapy, regardless of disease status. Consistent with the updated response criteria, lymphocytosis does not appear to impact the efficacy of IDELA in this pt population. Lymphocytosis also did not predispose pts to severe or unexpected AEs.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Lymphocyte, Progression, Targeted therapy
Abstract: E1067
Type: Eposter Presentation
Background
Lymphocytosis is a known effect of B-cell receptor (BCR) targeted therapies used to treat CLL and SLL, including idelalisib (IDELA). IDELA is a selective oral PI3Kδ inhibitor which inhibits multiple signaling pathways, including those downstream of the B-cell receptor, CXCR4, and CXCR5. Per updated response criteria (Hallek M, el al. Blood. 2012;119:5348; Cheson B, et al. J Clin Oncol. 2012;30:2820-2822.), drug-induced lymphocytosis alone should not interfere with the time of designation of a partial response (PR) and is not considered progressive disease (PD) in the absence of other signs or symptoms of progression.
Aims
This post hoc analysis characterized the patterns of lymphocytosis observed in patients (pts) with CLL/SLL treated with IDELA-based regimens, and evaluated the effect of lymphocytosis on safety and efficacy.
Methods
Pts from 4 studies (2 phase 1, 1 phase 2 and 1 phase 3) who received IDELA dosed at 100 or 150 mg BID were included in this analysis. Pts were grouped by disease status (treatment-naïve vs relapsed/refractory) and treatment regimen (IDELA-monotherapy vs IDELA in combination with chemotherapy, an anti-CD20 mAb [anti-CD20], or both. Chemotherapeutic agents included chlorambucil, bendamustine, or fludarabine. Anti-CD20 mAbs included rituximab or ofatumumab. Absolute lymphocyte counts (ALC) were measured throughout each study to calculate peak ALC, time to peak ALC, and time to 50% reduction from baseline ALC. To evaluate the effect of lymphocytosis on safety, grade ≥3 treatment-emergent adverse events (TEAEs) and TEAEs of interest (leukostasis, blood viscosity abnormalities, central nervous system hemorrhage, and disseminated intravascular coagulation) were summarized in pts with ALC >200 K/µl at any time during IDELA treatment. To evaluate the effect of lymphocytosis on efficacy, median progression-free survival (PFS) was analyzed in pts receiving IDELA monotherapy for relapsed/refractory CLL (phase 1 study) and treatment-naive CLL/SLL (phase 2 study) stratified by ALC reduction from baseline (≥50% [yes or no]).
Results
The analysis population included 352 pts (105 treatment-naïve; 247 relapsed/refractory), 63 on IDELA monotherapy and 289 on IDELA combination regimens. 68% of pts experienced a post-baseline increase in ALC, including 83% of pts on IDELA monotherapy and 50% of pts on combinations. Time to peak was 2-4 weeks across groups. 73% of pts achieved a 50% reduction from baseline ALC, including 33% of pts on IDELA monotherapy and 82% of pts on combinations. Median time to 50% reduction from baseline ALC was longest for pts with relapsed/refractory CLL/SLL on IDELA monotherapy (15 wks) and shortest for pts with relapsed/refractory CLL on IDELA+chemotherapy+anti-CD20 (2 wks). The safety analysis included 31/352 pts (9%) with ALC >200 K/µl at any time on IDELA treatment, with a maximum reported ALC of 447 K/µl. Of these 31 pts, Grade 3/4 TEAEs were reported in 8 (26%) during the time when ALC >200 K/µl. No pt with signs or symptoms of hyperleukocytosis was identified.The efficacy analysis included 63 pts (41 treatment-naïve; 22 relapsed/refractory) on IDELA-monotherapy. The median PFS was similar for pts with or without a ≥50% reduction from baseline ALC (Table).
Conclusion
Addition of anti-CD20 and/or chemotherapy to IDELA abrogated the transient lymphocytosis seen with IDELA-monotherapy, regardless of disease status. Consistent with the updated response criteria, lymphocytosis does not appear to impact the efficacy of IDELA in this pt population. Lymphocytosis also did not predispose pts to severe or unexpected AEs.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Lymphocyte, Progression, Targeted therapy
Type: Eposter Presentation
Background
Lymphocytosis is a known effect of B-cell receptor (BCR) targeted therapies used to treat CLL and SLL, including idelalisib (IDELA). IDELA is a selective oral PI3Kδ inhibitor which inhibits multiple signaling pathways, including those downstream of the B-cell receptor, CXCR4, and CXCR5. Per updated response criteria (Hallek M, el al. Blood. 2012;119:5348; Cheson B, et al. J Clin Oncol. 2012;30:2820-2822.), drug-induced lymphocytosis alone should not interfere with the time of designation of a partial response (PR) and is not considered progressive disease (PD) in the absence of other signs or symptoms of progression.
Aims
This post hoc analysis characterized the patterns of lymphocytosis observed in patients (pts) with CLL/SLL treated with IDELA-based regimens, and evaluated the effect of lymphocytosis on safety and efficacy.
Methods
Pts from 4 studies (2 phase 1, 1 phase 2 and 1 phase 3) who received IDELA dosed at 100 or 150 mg BID were included in this analysis. Pts were grouped by disease status (treatment-naïve vs relapsed/refractory) and treatment regimen (IDELA-monotherapy vs IDELA in combination with chemotherapy, an anti-CD20 mAb [anti-CD20], or both. Chemotherapeutic agents included chlorambucil, bendamustine, or fludarabine. Anti-CD20 mAbs included rituximab or ofatumumab. Absolute lymphocyte counts (ALC) were measured throughout each study to calculate peak ALC, time to peak ALC, and time to 50% reduction from baseline ALC. To evaluate the effect of lymphocytosis on safety, grade ≥3 treatment-emergent adverse events (TEAEs) and TEAEs of interest (leukostasis, blood viscosity abnormalities, central nervous system hemorrhage, and disseminated intravascular coagulation) were summarized in pts with ALC >200 K/µl at any time during IDELA treatment. To evaluate the effect of lymphocytosis on efficacy, median progression-free survival (PFS) was analyzed in pts receiving IDELA monotherapy for relapsed/refractory CLL (phase 1 study) and treatment-naive CLL/SLL (phase 2 study) stratified by ALC reduction from baseline (≥50% [yes or no]).
Results
The analysis population included 352 pts (105 treatment-naïve; 247 relapsed/refractory), 63 on IDELA monotherapy and 289 on IDELA combination regimens. 68% of pts experienced a post-baseline increase in ALC, including 83% of pts on IDELA monotherapy and 50% of pts on combinations. Time to peak was 2-4 weeks across groups. 73% of pts achieved a 50% reduction from baseline ALC, including 33% of pts on IDELA monotherapy and 82% of pts on combinations. Median time to 50% reduction from baseline ALC was longest for pts with relapsed/refractory CLL/SLL on IDELA monotherapy (15 wks) and shortest for pts with relapsed/refractory CLL on IDELA+chemotherapy+anti-CD20 (2 wks). The safety analysis included 31/352 pts (9%) with ALC >200 K/µl at any time on IDELA treatment, with a maximum reported ALC of 447 K/µl. Of these 31 pts, Grade 3/4 TEAEs were reported in 8 (26%) during the time when ALC >200 K/µl. No pt with signs or symptoms of hyperleukocytosis was identified.The efficacy analysis included 63 pts (41 treatment-naïve; 22 relapsed/refractory) on IDELA-monotherapy. The median PFS was similar for pts with or without a ≥50% reduction from baseline ALC (Table).
Conclusion
Addition of anti-CD20 and/or chemotherapy to IDELA abrogated the transient lymphocytosis seen with IDELA-monotherapy, regardless of disease status. Consistent with the updated response criteria, lymphocytosis does not appear to impact the efficacy of IDELA in this pt population. Lymphocytosis also did not predispose pts to severe or unexpected AEs.

Session topic: E-poster
Keyword(s): Chronic lymphocytic leukemia, Lymphocyte, Progression, Targeted therapy
{{ help_message }}
{{filter}}


