SUBCUTANEOUS VERSUS INTRAVENOUS RITUXIMAB ADMINISTRATION IN FIRST-LINE DIFFUSE LARGE B-CELL LYMPHOMA AND FOLLICULAR LYMPHOMA: PREFMAB STUDY OF PATIENT PREFERENCE AND SATISFACTION IN 19 COUNTRIES
(Abstract release date: 05/19/16)
EHA Library. Rummel M. 06/09/16; 132505; E956

Prof. Mathias Rummel
Contributions
Contributions
Abstract
Abstract: E956
Type: Eposter Presentation
Background
A subcutaneous (SC) formulation of rituximab has been developed with comparable clinical activity to rituximab intravenous (IV), but a shorter administration time (~5 min vs 1.5-6 hrs). The SC administration route has been shown to reduce healthcare resource burden and has potential to reduce patient treatment burden compared with rituximab IV. In PrefMab, patients preferred rituximab SC over IV when given with chemotherapy, and reported higher levels of convenience and satisfaction with the rituximab SC administration route. However, in a large, international study, regional differences in patient preference due to local variations in clinical practice or culture may not be clear in the overall data.
Aims
To evaluate country-specific patient preference and satisfaction with rituximab SC vs IV.
Methods
PrefMab (NCT01724021) is a randomized, open-label, crossover phase 3b study. Patients were aged 18-80 years and had: previously untreated CD20+ diffuse large B-cell lymphoma (DLBCL; international prognostic index [IPI] 1-4 or 0 with bulky disease) or follicular lymphoma (FL; FLIPI grade 1-3a); at least 1 bi-dimensionally measurable lesion ≥1.5cm at its largest dimension; and ECOG performance status ≤3. All patients gave informed consent. Patients received 8 cycles of rituximab according to 2 schedules: 1 cycle rituximab IV (375mg/m2) and 3 cycles rituximab SC (1400mg) then 4 cycles rituximab IV; or 4 cycles rituximab IV (375mg/m2) then 4 cycles rituximab SC (1400mg). Patients also received 6-8 cycles of chemotherapy (CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone], CVP [cyclophosphamide, vincristine, prednisone], or bendamustine as per standard local practice). A Patient Preference Questionnaire (PPQ) was completed at cycles 6 and 8. A Rituximab Administration Satisfaction Questionnaire (RASQ) was completed at cycles 4 and 8; domains were scored 0 (least)-100 (best). The current analysis included countries with data for ≥10 patients.
Results
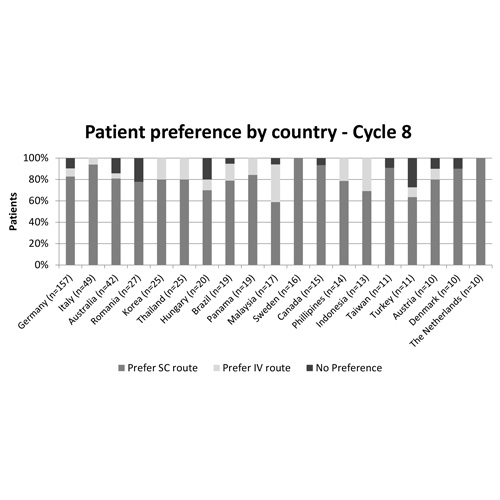
At the primary data cut-off (January 19, 2015), 743 patients had been randomized to treatment in 32 countries. The median age was 60 years, most patients had DLBCL (63% vs 37% FL) and baseline characteristics were balanced between arms. Nineteen countries (listed in Figure) with data available for ≥10 patients were eligible for and included in the analysis. At cycle 8, most patients preferred rituximab SC to IV, with the median score ranging from 60-100% (Figure); similar results were seen at cycle 6. Preference was not substantially impacted by underlying disease. Patient satisfaction was higher for the SC administration route. Compared with the IV route, median RASQ scores were ≥10 points higher for SC in: 0/19 countries in the physical impact domain; 8/19 countries in the psychological impact domain; 16/19 countries in the impact on activities of daily living domain; 15/19 countries in the convenience domain; and 14/19 countries in the satisfaction domain. In 18/19 countries most patients (>70%) thought the time taken to administer rituximab SC was 'just right', although 53% of patients in Brazil felt it was 'too short'. In all countries, most patients (>50%) felt they had 'more than enough time' to discuss their illness with their nurse and/or doctor, regardless of administration route, although in Germany, Brazil and Canada >50% patients felt the SC route impacted on this time.
Conclusion
Patient preference and satisfaction was higher with rituximab SC vs IV, and did not vary substantially by the country in which treatment was received.
Session topic: E-poster
Keyword(s): Diffuse large B cell lymphoma, Follicular lymphoma, Rituximab, Subcutaneous
Type: Eposter Presentation
Background
A subcutaneous (SC) formulation of rituximab has been developed with comparable clinical activity to rituximab intravenous (IV), but a shorter administration time (~5 min vs 1.5-6 hrs). The SC administration route has been shown to reduce healthcare resource burden and has potential to reduce patient treatment burden compared with rituximab IV. In PrefMab, patients preferred rituximab SC over IV when given with chemotherapy, and reported higher levels of convenience and satisfaction with the rituximab SC administration route. However, in a large, international study, regional differences in patient preference due to local variations in clinical practice or culture may not be clear in the overall data.
Aims
To evaluate country-specific patient preference and satisfaction with rituximab SC vs IV.
Methods
PrefMab (NCT01724021) is a randomized, open-label, crossover phase 3b study. Patients were aged 18-80 years and had: previously untreated CD20+ diffuse large B-cell lymphoma (DLBCL; international prognostic index [IPI] 1-4 or 0 with bulky disease) or follicular lymphoma (FL; FLIPI grade 1-3a); at least 1 bi-dimensionally measurable lesion ≥1.5cm at its largest dimension; and ECOG performance status ≤3. All patients gave informed consent. Patients received 8 cycles of rituximab according to 2 schedules: 1 cycle rituximab IV (375mg/m2) and 3 cycles rituximab SC (1400mg) then 4 cycles rituximab IV; or 4 cycles rituximab IV (375mg/m2) then 4 cycles rituximab SC (1400mg). Patients also received 6-8 cycles of chemotherapy (CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone], CVP [cyclophosphamide, vincristine, prednisone], or bendamustine as per standard local practice). A Patient Preference Questionnaire (PPQ) was completed at cycles 6 and 8. A Rituximab Administration Satisfaction Questionnaire (RASQ) was completed at cycles 4 and 8; domains were scored 0 (least)-100 (best). The current analysis included countries with data for ≥10 patients.
Results
At the primary data cut-off (January 19, 2015), 743 patients had been randomized to treatment in 32 countries. The median age was 60 years, most patients had DLBCL (63% vs 37% FL) and baseline characteristics were balanced between arms. Nineteen countries (listed in Figure) with data available for ≥10 patients were eligible for and included in the analysis. At cycle 8, most patients preferred rituximab SC to IV, with the median score ranging from 60-100% (Figure); similar results were seen at cycle 6. Preference was not substantially impacted by underlying disease. Patient satisfaction was higher for the SC administration route. Compared with the IV route, median RASQ scores were ≥10 points higher for SC in: 0/19 countries in the physical impact domain; 8/19 countries in the psychological impact domain; 16/19 countries in the impact on activities of daily living domain; 15/19 countries in the convenience domain; and 14/19 countries in the satisfaction domain. In 18/19 countries most patients (>70%) thought the time taken to administer rituximab SC was 'just right', although 53% of patients in Brazil felt it was 'too short'. In all countries, most patients (>50%) felt they had 'more than enough time' to discuss their illness with their nurse and/or doctor, regardless of administration route, although in Germany, Brazil and Canada >50% patients felt the SC route impacted on this time.
Conclusion
Patient preference and satisfaction was higher with rituximab SC vs IV, and did not vary substantially by the country in which treatment was received.

Session topic: E-poster
Keyword(s): Diffuse large B cell lymphoma, Follicular lymphoma, Rituximab, Subcutaneous
Abstract: E956
Type: Eposter Presentation
Background
A subcutaneous (SC) formulation of rituximab has been developed with comparable clinical activity to rituximab intravenous (IV), but a shorter administration time (~5 min vs 1.5-6 hrs). The SC administration route has been shown to reduce healthcare resource burden and has potential to reduce patient treatment burden compared with rituximab IV. In PrefMab, patients preferred rituximab SC over IV when given with chemotherapy, and reported higher levels of convenience and satisfaction with the rituximab SC administration route. However, in a large, international study, regional differences in patient preference due to local variations in clinical practice or culture may not be clear in the overall data.
Aims
To evaluate country-specific patient preference and satisfaction with rituximab SC vs IV.
Methods
PrefMab (NCT01724021) is a randomized, open-label, crossover phase 3b study. Patients were aged 18-80 years and had: previously untreated CD20+ diffuse large B-cell lymphoma (DLBCL; international prognostic index [IPI] 1-4 or 0 with bulky disease) or follicular lymphoma (FL; FLIPI grade 1-3a); at least 1 bi-dimensionally measurable lesion ≥1.5cm at its largest dimension; and ECOG performance status ≤3. All patients gave informed consent. Patients received 8 cycles of rituximab according to 2 schedules: 1 cycle rituximab IV (375mg/m2) and 3 cycles rituximab SC (1400mg) then 4 cycles rituximab IV; or 4 cycles rituximab IV (375mg/m2) then 4 cycles rituximab SC (1400mg). Patients also received 6-8 cycles of chemotherapy (CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone], CVP [cyclophosphamide, vincristine, prednisone], or bendamustine as per standard local practice). A Patient Preference Questionnaire (PPQ) was completed at cycles 6 and 8. A Rituximab Administration Satisfaction Questionnaire (RASQ) was completed at cycles 4 and 8; domains were scored 0 (least)-100 (best). The current analysis included countries with data for ≥10 patients.
Results
At the primary data cut-off (January 19, 2015), 743 patients had been randomized to treatment in 32 countries. The median age was 60 years, most patients had DLBCL (63% vs 37% FL) and baseline characteristics were balanced between arms. Nineteen countries (listed in Figure) with data available for ≥10 patients were eligible for and included in the analysis. At cycle 8, most patients preferred rituximab SC to IV, with the median score ranging from 60-100% (Figure); similar results were seen at cycle 6. Preference was not substantially impacted by underlying disease. Patient satisfaction was higher for the SC administration route. Compared with the IV route, median RASQ scores were ≥10 points higher for SC in: 0/19 countries in the physical impact domain; 8/19 countries in the psychological impact domain; 16/19 countries in the impact on activities of daily living domain; 15/19 countries in the convenience domain; and 14/19 countries in the satisfaction domain. In 18/19 countries most patients (>70%) thought the time taken to administer rituximab SC was 'just right', although 53% of patients in Brazil felt it was 'too short'. In all countries, most patients (>50%) felt they had 'more than enough time' to discuss their illness with their nurse and/or doctor, regardless of administration route, although in Germany, Brazil and Canada >50% patients felt the SC route impacted on this time.
Conclusion
Patient preference and satisfaction was higher with rituximab SC vs IV, and did not vary substantially by the country in which treatment was received.

Session topic: E-poster
Keyword(s): Diffuse large B cell lymphoma, Follicular lymphoma, Rituximab, Subcutaneous
Type: Eposter Presentation
Background
A subcutaneous (SC) formulation of rituximab has been developed with comparable clinical activity to rituximab intravenous (IV), but a shorter administration time (~5 min vs 1.5-6 hrs). The SC administration route has been shown to reduce healthcare resource burden and has potential to reduce patient treatment burden compared with rituximab IV. In PrefMab, patients preferred rituximab SC over IV when given with chemotherapy, and reported higher levels of convenience and satisfaction with the rituximab SC administration route. However, in a large, international study, regional differences in patient preference due to local variations in clinical practice or culture may not be clear in the overall data.
Aims
To evaluate country-specific patient preference and satisfaction with rituximab SC vs IV.
Methods
PrefMab (NCT01724021) is a randomized, open-label, crossover phase 3b study. Patients were aged 18-80 years and had: previously untreated CD20+ diffuse large B-cell lymphoma (DLBCL; international prognostic index [IPI] 1-4 or 0 with bulky disease) or follicular lymphoma (FL; FLIPI grade 1-3a); at least 1 bi-dimensionally measurable lesion ≥1.5cm at its largest dimension; and ECOG performance status ≤3. All patients gave informed consent. Patients received 8 cycles of rituximab according to 2 schedules: 1 cycle rituximab IV (375mg/m2) and 3 cycles rituximab SC (1400mg) then 4 cycles rituximab IV; or 4 cycles rituximab IV (375mg/m2) then 4 cycles rituximab SC (1400mg). Patients also received 6-8 cycles of chemotherapy (CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone], CVP [cyclophosphamide, vincristine, prednisone], or bendamustine as per standard local practice). A Patient Preference Questionnaire (PPQ) was completed at cycles 6 and 8. A Rituximab Administration Satisfaction Questionnaire (RASQ) was completed at cycles 4 and 8; domains were scored 0 (least)-100 (best). The current analysis included countries with data for ≥10 patients.
Results
At the primary data cut-off (January 19, 2015), 743 patients had been randomized to treatment in 32 countries. The median age was 60 years, most patients had DLBCL (63% vs 37% FL) and baseline characteristics were balanced between arms. Nineteen countries (listed in Figure) with data available for ≥10 patients were eligible for and included in the analysis. At cycle 8, most patients preferred rituximab SC to IV, with the median score ranging from 60-100% (Figure); similar results were seen at cycle 6. Preference was not substantially impacted by underlying disease. Patient satisfaction was higher for the SC administration route. Compared with the IV route, median RASQ scores were ≥10 points higher for SC in: 0/19 countries in the physical impact domain; 8/19 countries in the psychological impact domain; 16/19 countries in the impact on activities of daily living domain; 15/19 countries in the convenience domain; and 14/19 countries in the satisfaction domain. In 18/19 countries most patients (>70%) thought the time taken to administer rituximab SC was 'just right', although 53% of patients in Brazil felt it was 'too short'. In all countries, most patients (>50%) felt they had 'more than enough time' to discuss their illness with their nurse and/or doctor, regardless of administration route, although in Germany, Brazil and Canada >50% patients felt the SC route impacted on this time.
Conclusion
Patient preference and satisfaction was higher with rituximab SC vs IV, and did not vary substantially by the country in which treatment was received.

Session topic: E-poster
Keyword(s): Diffuse large B cell lymphoma, Follicular lymphoma, Rituximab, Subcutaneous
{{ help_message }}
{{filter}}


