SPIRIT 2: AN NCRI RANDOMISED STUDY COMPARING DASATINIB WITH IMATINIB IN PATIENTS WITH NEWLY-DIAGNOSED CHRONIC MYELOID LEUKAEMIA ? 2 YEAR FOLLOW UP
(Abstract release date: 05/21/15)
EHA Library. O'Brien S. 06/13/15; 103232; S489
Disclosure(s): Newcastle UniversityNICR

Stephen O'Brien
Contributions
Contributions
Abstract
Abstract: S489
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 16:30 to 13.06.2015 16:45
Location: Room A8
Background
SPIRIT 2 is the largest trial comparing imatinib 400mg with dasatinib 100mg daily.
Aims
The primary end point of the study is event-free survival at 5 years. A key secondary endpoint is rate of a major molecular response (MMR, MR3, BCR-ABL1/ABL ratio <0.1% international scale).
Methods
814 patients were recruited from 144 hospitals in the UK between August 2008 and March 2013. 812 patients were randomised, 406 in each arm.
Results
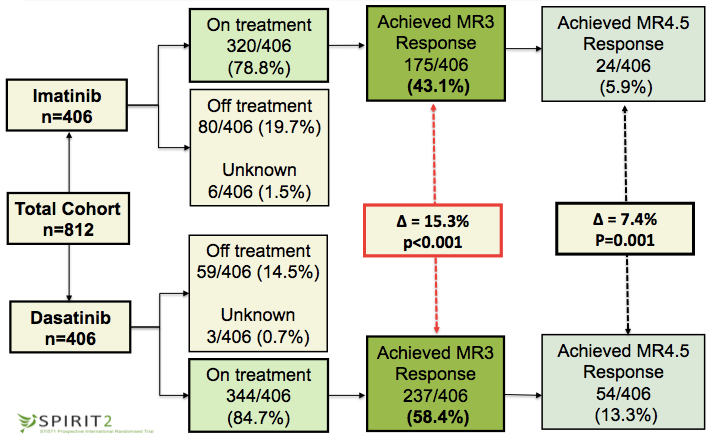
With a median follow up of 37.4 months, 236 imatinib and 276 dasatinib patients still received study treatment. 133 patients discontinued due to non-haematological toxicity. 53/406 (13.1%) on the imatinib arm (predominantly gastrointestinal toxicity) and 80/406 (19.7%) dasatinib patients (predominantly thrombocytopenia). 45/814 patients discontinued due to sub-optimal response as assessed by the treating physician (not defined by the protocol). These were predominantly patients on imatinib (42 of 406, 10.3%) as compared to dasatinib - 3 of 406, 0.7%. Pleural effusions occurred in 90/406 (22.2%) patients on dasatinib and 13 required drainage. There was no significant difference in arterial cardiovascular events: imatinib 3/406 (0.7%); dasatinib 9/406 (2.2%). The MR3 rate at 12 months was significantly (p<0.001) higher with dasatinib 237/406 (58.4%) compared to imatinib 175/406 (43.1%) patients. In contrast to previous studies, pleural effusion was not associated with a significantly higher rate of MR3. Accelerated phase (imatinib 1/406; dasatinib 2/406) and blast crisis (imatinib 7/406; dasatinib 4/406) were observed in 14 patients. 38 patients died and there was no significant difference in deat rate in the two arms.
Summary
In conclusion, dasatinib-treated patients have a significantly higher rate of molecular response at 1 year but so far there is no significant difference in rates of disease progression or overall survival. More patients abandoned imatinib than dasatinib due to investigator and/or patient concerns about sub-optimal responses. Further follow up will evaluate differences in the primary endpoint: event free survival at five years.
Keyword(s): Chronic myeloid leukemia, Imatinib
Session topic: CML: Clinical trials
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 16:30 to 13.06.2015 16:45
Location: Room A8
Background
SPIRIT 2 is the largest trial comparing imatinib 400mg with dasatinib 100mg daily.
Aims
The primary end point of the study is event-free survival at 5 years. A key secondary endpoint is rate of a major molecular response (MMR, MR3, BCR-ABL1/ABL ratio <0.1% international scale).
Methods
814 patients were recruited from 144 hospitals in the UK between August 2008 and March 2013. 812 patients were randomised, 406 in each arm.
Results
With a median follow up of 37.4 months, 236 imatinib and 276 dasatinib patients still received study treatment. 133 patients discontinued due to non-haematological toxicity. 53/406 (13.1%) on the imatinib arm (predominantly gastrointestinal toxicity) and 80/406 (19.7%) dasatinib patients (predominantly thrombocytopenia). 45/814 patients discontinued due to sub-optimal response as assessed by the treating physician (not defined by the protocol). These were predominantly patients on imatinib (42 of 406, 10.3%) as compared to dasatinib - 3 of 406, 0.7%. Pleural effusions occurred in 90/406 (22.2%) patients on dasatinib and 13 required drainage. There was no significant difference in arterial cardiovascular events: imatinib 3/406 (0.7%); dasatinib 9/406 (2.2%). The MR3 rate at 12 months was significantly (p<0.001) higher with dasatinib 237/406 (58.4%) compared to imatinib 175/406 (43.1%) patients. In contrast to previous studies, pleural effusion was not associated with a significantly higher rate of MR3. Accelerated phase (imatinib 1/406; dasatinib 2/406) and blast crisis (imatinib 7/406; dasatinib 4/406) were observed in 14 patients. 38 patients died and there was no significant difference in deat rate in the two arms.
Summary
In conclusion, dasatinib-treated patients have a significantly higher rate of molecular response at 1 year but so far there is no significant difference in rates of disease progression or overall survival. More patients abandoned imatinib than dasatinib due to investigator and/or patient concerns about sub-optimal responses. Further follow up will evaluate differences in the primary endpoint: event free survival at five years.
Keyword(s): Chronic myeloid leukemia, Imatinib

Session topic: CML: Clinical trials
Abstract: S489
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 16:30 to 13.06.2015 16:45
Location: Room A8
Background
SPIRIT 2 is the largest trial comparing imatinib 400mg with dasatinib 100mg daily.
Aims
The primary end point of the study is event-free survival at 5 years. A key secondary endpoint is rate of a major molecular response (MMR, MR3, BCR-ABL1/ABL ratio <0.1% international scale).
Methods
814 patients were recruited from 144 hospitals in the UK between August 2008 and March 2013. 812 patients were randomised, 406 in each arm.
Results
With a median follow up of 37.4 months, 236 imatinib and 276 dasatinib patients still received study treatment. 133 patients discontinued due to non-haematological toxicity. 53/406 (13.1%) on the imatinib arm (predominantly gastrointestinal toxicity) and 80/406 (19.7%) dasatinib patients (predominantly thrombocytopenia). 45/814 patients discontinued due to sub-optimal response as assessed by the treating physician (not defined by the protocol). These were predominantly patients on imatinib (42 of 406, 10.3%) as compared to dasatinib - 3 of 406, 0.7%. Pleural effusions occurred in 90/406 (22.2%) patients on dasatinib and 13 required drainage. There was no significant difference in arterial cardiovascular events: imatinib 3/406 (0.7%); dasatinib 9/406 (2.2%). The MR3 rate at 12 months was significantly (p<0.001) higher with dasatinib 237/406 (58.4%) compared to imatinib 175/406 (43.1%) patients. In contrast to previous studies, pleural effusion was not associated with a significantly higher rate of MR3. Accelerated phase (imatinib 1/406; dasatinib 2/406) and blast crisis (imatinib 7/406; dasatinib 4/406) were observed in 14 patients. 38 patients died and there was no significant difference in deat rate in the two arms.
Summary
In conclusion, dasatinib-treated patients have a significantly higher rate of molecular response at 1 year but so far there is no significant difference in rates of disease progression or overall survival. More patients abandoned imatinib than dasatinib due to investigator and/or patient concerns about sub-optimal responses. Further follow up will evaluate differences in the primary endpoint: event free survival at five years.
Keyword(s): Chronic myeloid leukemia, Imatinib

Session topic: CML: Clinical trials
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 16:30 to 13.06.2015 16:45
Location: Room A8
Background
SPIRIT 2 is the largest trial comparing imatinib 400mg with dasatinib 100mg daily.
Aims
The primary end point of the study is event-free survival at 5 years. A key secondary endpoint is rate of a major molecular response (MMR, MR3, BCR-ABL1/ABL ratio <0.1% international scale).
Methods
814 patients were recruited from 144 hospitals in the UK between August 2008 and March 2013. 812 patients were randomised, 406 in each arm.
Results
With a median follow up of 37.4 months, 236 imatinib and 276 dasatinib patients still received study treatment. 133 patients discontinued due to non-haematological toxicity. 53/406 (13.1%) on the imatinib arm (predominantly gastrointestinal toxicity) and 80/406 (19.7%) dasatinib patients (predominantly thrombocytopenia). 45/814 patients discontinued due to sub-optimal response as assessed by the treating physician (not defined by the protocol). These were predominantly patients on imatinib (42 of 406, 10.3%) as compared to dasatinib - 3 of 406, 0.7%. Pleural effusions occurred in 90/406 (22.2%) patients on dasatinib and 13 required drainage. There was no significant difference in arterial cardiovascular events: imatinib 3/406 (0.7%); dasatinib 9/406 (2.2%). The MR3 rate at 12 months was significantly (p<0.001) higher with dasatinib 237/406 (58.4%) compared to imatinib 175/406 (43.1%) patients. In contrast to previous studies, pleural effusion was not associated with a significantly higher rate of MR3. Accelerated phase (imatinib 1/406; dasatinib 2/406) and blast crisis (imatinib 7/406; dasatinib 4/406) were observed in 14 patients. 38 patients died and there was no significant difference in deat rate in the two arms.
Summary
In conclusion, dasatinib-treated patients have a significantly higher rate of molecular response at 1 year but so far there is no significant difference in rates of disease progression or overall survival. More patients abandoned imatinib than dasatinib due to investigator and/or patient concerns about sub-optimal responses. Further follow up will evaluate differences in the primary endpoint: event free survival at five years.
Keyword(s): Chronic myeloid leukemia, Imatinib

Session topic: CML: Clinical trials
{{ help_message }}
{{filter}}


