SUBCUTANEOUS VERSUS INTRAVENOUS RITUXIMAB IN COMBINATION WITH CHOP FOR PREVIOUSLY UNTREATED DIFFUSE LARGE B-CELL LYMPHOMA: EFFICACY AND SAFETY RESULTS FROM THE PHASE IIIB MABEASE STUDY
(Abstract release date: 05/21/15)
EHA Library. Lugtenburg P. 06/13/15; 103114; S483
Disclosure(s): Erasmus MC Cancer Institute
Dr. Pieternella Lugtenburg
Contributions
Contributions
Abstract
Abstract: S483
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 16:15 to 13.06.2015 16:30
Location: Room A7
Background
Subcutaneous rituximab (RSC) offers improved patient (pt) convenience and healthcare resource savings versus intravenous R (RIV), with similar efficacy and safety outcomes in the SABRINA study in follicular lymphoma (FL). RSC is approved in Europe and other countries for use in FL and diffuse large B-cell lymphoma (DLBCL).
Aims
To confirm the efficacy and safety of RSC versus RIV plus CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) for the treatment of DLBCL.
Methods
The open-label, randomised MABEASE study (NCT01649856) investigated the efficacy of RSC versus RIV plus CHOP in untreated DLBCL. Pts were randomised 2:1 to receive RSC (RIV 375 mg/m2 cycle 1; RSC 1400 mg fixed dose cycles 2–8) or RIV (RIV 375 mg/m2 cycles 1–8), plus 6 or 8 cycles of CHOP every 14 or 21 days. Responses (complete response [CR], unconfirmed CR [CRu], partial response [PR], progressive disease [PD]) were determined by investigator assessment (Cheson 1999 criteria). Administration-related reactions (ARRs) were defined as R-related adverse events (AEs) that occurred ≤24 hours of administration. The primary endpoint was response rate at end of treatment (EOT). Safety was a secondary endpoint.
Results
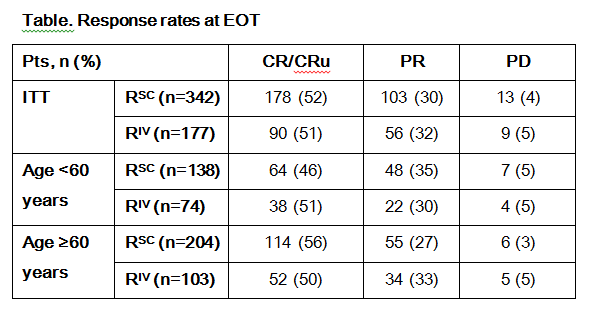
In total, 576 pts were randomised (RSC n=381; RIV n=195; intent-to-treat [ITT] population); baseline characteristics were balanced between arms. A total of 572 pts (RSC n=378; RIV n=194) received ≥1 dose of R. Nine RSC pts discontinued after cycle 1, due to AE (n=5), withdrawn consent (n=2), PD and protocol violation (both n=1); as both arms received RIV during cycle 1, these pts were included in the RIV arm for safety analyses (RSC n=369; RIV n=203). At EOT (RSC n=342; RIV n=177), response rates were similar between arms in the ITT population overall (RSC 52%; RIV 51%) and when pts were stratified by age (~50% for all groups; Table). AE rates were comparable between the RSC and RIV arms overall (92% vs 91%) and during cycle 1 (61% vs 64%); the most common AE overall was neutropaenia (RSC 36%; RIV 33%). During cycles 2–8, RSC and RIV pts had similar rates of grade ≥3 AEs (53% vs 50%) and serious AEs (SAEs; 34% vs 31%); the most frequent grade ≥3 AE was neutropaenia (RSC 22%; RIV 20%) and the most common SAE was febrile neutropaenia (RSC 11%; RIV 6%). ARR rates were similar between RSC and RIV pts overall (28%/arm), during cycle 1 (11% vs 14%) and cycles 2–8 (22% vs 20%). From cycle 2–8, the most common ARR by System Organ Class (SOC) was general disorders and administrative site conditions (RSC 10%; RIV 4%). Rates of grade ≥3 ARRs was similar between arms overall (2% vs 5%), during cycle 1 (0% vs 2%) and cycles 2–8 (2% vs 4%); from cycles 2–8, the most common grade ≥3 ARR by SOC was blood and lymphatic system disorders (RSC 1%; RIV 3%). In total, 52% (RSC) and 54% (RIV) of pts had R-related AEs; the most frequent R-related AE was neutropaenia (RSC 14%; RIV 16%). Overall, 8% (RSC) and 9% (RIV) of pts discontinued R due to AEs, most commonly infections (RSC 2%; RIV 3%). In total, 63 pts (11%) died (RSC 10%; RIV 13%). AEs led to death in 6% (RSC) and 7% (RIV) of pts, most commonly due to infections (2%/arm).
Summary
RSC and RIV plus CHOP had similar efficacy and safety outcomes in previously untreated DLBCL. RSC offers convenience for pts and efficient utilisation of healthcare resources.
Keyword(s): CHOP, Diffuse large B cell lymphoma, Rituximab
Session topic: Optimization and innovation in treating aggressive lymphomas
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 16:15 to 13.06.2015 16:30
Location: Room A7
Background
Subcutaneous rituximab (RSC) offers improved patient (pt) convenience and healthcare resource savings versus intravenous R (RIV), with similar efficacy and safety outcomes in the SABRINA study in follicular lymphoma (FL). RSC is approved in Europe and other countries for use in FL and diffuse large B-cell lymphoma (DLBCL).
Aims
To confirm the efficacy and safety of RSC versus RIV plus CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) for the treatment of DLBCL.
Methods
The open-label, randomised MABEASE study (NCT01649856) investigated the efficacy of RSC versus RIV plus CHOP in untreated DLBCL. Pts were randomised 2:1 to receive RSC (RIV 375 mg/m2 cycle 1; RSC 1400 mg fixed dose cycles 2–8) or RIV (RIV 375 mg/m2 cycles 1–8), plus 6 or 8 cycles of CHOP every 14 or 21 days. Responses (complete response [CR], unconfirmed CR [CRu], partial response [PR], progressive disease [PD]) were determined by investigator assessment (Cheson 1999 criteria). Administration-related reactions (ARRs) were defined as R-related adverse events (AEs) that occurred ≤24 hours of administration. The primary endpoint was response rate at end of treatment (EOT). Safety was a secondary endpoint.
Results
In total, 576 pts were randomised (RSC n=381; RIV n=195; intent-to-treat [ITT] population); baseline characteristics were balanced between arms. A total of 572 pts (RSC n=378; RIV n=194) received ≥1 dose of R. Nine RSC pts discontinued after cycle 1, due to AE (n=5), withdrawn consent (n=2), PD and protocol violation (both n=1); as both arms received RIV during cycle 1, these pts were included in the RIV arm for safety analyses (RSC n=369; RIV n=203). At EOT (RSC n=342; RIV n=177), response rates were similar between arms in the ITT population overall (RSC 52%; RIV 51%) and when pts were stratified by age (~50% for all groups; Table). AE rates were comparable between the RSC and RIV arms overall (92% vs 91%) and during cycle 1 (61% vs 64%); the most common AE overall was neutropaenia (RSC 36%; RIV 33%). During cycles 2–8, RSC and RIV pts had similar rates of grade ≥3 AEs (53% vs 50%) and serious AEs (SAEs; 34% vs 31%); the most frequent grade ≥3 AE was neutropaenia (RSC 22%; RIV 20%) and the most common SAE was febrile neutropaenia (RSC 11%; RIV 6%). ARR rates were similar between RSC and RIV pts overall (28%/arm), during cycle 1 (11% vs 14%) and cycles 2–8 (22% vs 20%). From cycle 2–8, the most common ARR by System Organ Class (SOC) was general disorders and administrative site conditions (RSC 10%; RIV 4%). Rates of grade ≥3 ARRs was similar between arms overall (2% vs 5%), during cycle 1 (0% vs 2%) and cycles 2–8 (2% vs 4%); from cycles 2–8, the most common grade ≥3 ARR by SOC was blood and lymphatic system disorders (RSC 1%; RIV 3%). In total, 52% (RSC) and 54% (RIV) of pts had R-related AEs; the most frequent R-related AE was neutropaenia (RSC 14%; RIV 16%). Overall, 8% (RSC) and 9% (RIV) of pts discontinued R due to AEs, most commonly infections (RSC 2%; RIV 3%). In total, 63 pts (11%) died (RSC 10%; RIV 13%). AEs led to death in 6% (RSC) and 7% (RIV) of pts, most commonly due to infections (2%/arm).
Summary
RSC and RIV plus CHOP had similar efficacy and safety outcomes in previously untreated DLBCL. RSC offers convenience for pts and efficient utilisation of healthcare resources.
Keyword(s): CHOP, Diffuse large B cell lymphoma, Rituximab

Session topic: Optimization and innovation in treating aggressive lymphomas
Abstract: S483
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 16:15 to 13.06.2015 16:30
Location: Room A7
Background
Subcutaneous rituximab (RSC) offers improved patient (pt) convenience and healthcare resource savings versus intravenous R (RIV), with similar efficacy and safety outcomes in the SABRINA study in follicular lymphoma (FL). RSC is approved in Europe and other countries for use in FL and diffuse large B-cell lymphoma (DLBCL).
Aims
To confirm the efficacy and safety of RSC versus RIV plus CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) for the treatment of DLBCL.
Methods
The open-label, randomised MABEASE study (NCT01649856) investigated the efficacy of RSC versus RIV plus CHOP in untreated DLBCL. Pts were randomised 2:1 to receive RSC (RIV 375 mg/m2 cycle 1; RSC 1400 mg fixed dose cycles 2–8) or RIV (RIV 375 mg/m2 cycles 1–8), plus 6 or 8 cycles of CHOP every 14 or 21 days. Responses (complete response [CR], unconfirmed CR [CRu], partial response [PR], progressive disease [PD]) were determined by investigator assessment (Cheson 1999 criteria). Administration-related reactions (ARRs) were defined as R-related adverse events (AEs) that occurred ≤24 hours of administration. The primary endpoint was response rate at end of treatment (EOT). Safety was a secondary endpoint.
Results
In total, 576 pts were randomised (RSC n=381; RIV n=195; intent-to-treat [ITT] population); baseline characteristics were balanced between arms. A total of 572 pts (RSC n=378; RIV n=194) received ≥1 dose of R. Nine RSC pts discontinued after cycle 1, due to AE (n=5), withdrawn consent (n=2), PD and protocol violation (both n=1); as both arms received RIV during cycle 1, these pts were included in the RIV arm for safety analyses (RSC n=369; RIV n=203). At EOT (RSC n=342; RIV n=177), response rates were similar between arms in the ITT population overall (RSC 52%; RIV 51%) and when pts were stratified by age (~50% for all groups; Table). AE rates were comparable between the RSC and RIV arms overall (92% vs 91%) and during cycle 1 (61% vs 64%); the most common AE overall was neutropaenia (RSC 36%; RIV 33%). During cycles 2–8, RSC and RIV pts had similar rates of grade ≥3 AEs (53% vs 50%) and serious AEs (SAEs; 34% vs 31%); the most frequent grade ≥3 AE was neutropaenia (RSC 22%; RIV 20%) and the most common SAE was febrile neutropaenia (RSC 11%; RIV 6%). ARR rates were similar between RSC and RIV pts overall (28%/arm), during cycle 1 (11% vs 14%) and cycles 2–8 (22% vs 20%). From cycle 2–8, the most common ARR by System Organ Class (SOC) was general disorders and administrative site conditions (RSC 10%; RIV 4%). Rates of grade ≥3 ARRs was similar between arms overall (2% vs 5%), during cycle 1 (0% vs 2%) and cycles 2–8 (2% vs 4%); from cycles 2–8, the most common grade ≥3 ARR by SOC was blood and lymphatic system disorders (RSC 1%; RIV 3%). In total, 52% (RSC) and 54% (RIV) of pts had R-related AEs; the most frequent R-related AE was neutropaenia (RSC 14%; RIV 16%). Overall, 8% (RSC) and 9% (RIV) of pts discontinued R due to AEs, most commonly infections (RSC 2%; RIV 3%). In total, 63 pts (11%) died (RSC 10%; RIV 13%). AEs led to death in 6% (RSC) and 7% (RIV) of pts, most commonly due to infections (2%/arm).
Summary
RSC and RIV plus CHOP had similar efficacy and safety outcomes in previously untreated DLBCL. RSC offers convenience for pts and efficient utilisation of healthcare resources.
Keyword(s): CHOP, Diffuse large B cell lymphoma, Rituximab

Session topic: Optimization and innovation in treating aggressive lymphomas
Type: Oral Presentation
Presentation during EHA20: From 13.06.2015 16:15 to 13.06.2015 16:30
Location: Room A7
Background
Subcutaneous rituximab (RSC) offers improved patient (pt) convenience and healthcare resource savings versus intravenous R (RIV), with similar efficacy and safety outcomes in the SABRINA study in follicular lymphoma (FL). RSC is approved in Europe and other countries for use in FL and diffuse large B-cell lymphoma (DLBCL).
Aims
To confirm the efficacy and safety of RSC versus RIV plus CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) for the treatment of DLBCL.
Methods
The open-label, randomised MABEASE study (NCT01649856) investigated the efficacy of RSC versus RIV plus CHOP in untreated DLBCL. Pts were randomised 2:1 to receive RSC (RIV 375 mg/m2 cycle 1; RSC 1400 mg fixed dose cycles 2–8) or RIV (RIV 375 mg/m2 cycles 1–8), plus 6 or 8 cycles of CHOP every 14 or 21 days. Responses (complete response [CR], unconfirmed CR [CRu], partial response [PR], progressive disease [PD]) were determined by investigator assessment (Cheson 1999 criteria). Administration-related reactions (ARRs) were defined as R-related adverse events (AEs) that occurred ≤24 hours of administration. The primary endpoint was response rate at end of treatment (EOT). Safety was a secondary endpoint.
Results
In total, 576 pts were randomised (RSC n=381; RIV n=195; intent-to-treat [ITT] population); baseline characteristics were balanced between arms. A total of 572 pts (RSC n=378; RIV n=194) received ≥1 dose of R. Nine RSC pts discontinued after cycle 1, due to AE (n=5), withdrawn consent (n=2), PD and protocol violation (both n=1); as both arms received RIV during cycle 1, these pts were included in the RIV arm for safety analyses (RSC n=369; RIV n=203). At EOT (RSC n=342; RIV n=177), response rates were similar between arms in the ITT population overall (RSC 52%; RIV 51%) and when pts were stratified by age (~50% for all groups; Table). AE rates were comparable between the RSC and RIV arms overall (92% vs 91%) and during cycle 1 (61% vs 64%); the most common AE overall was neutropaenia (RSC 36%; RIV 33%). During cycles 2–8, RSC and RIV pts had similar rates of grade ≥3 AEs (53% vs 50%) and serious AEs (SAEs; 34% vs 31%); the most frequent grade ≥3 AE was neutropaenia (RSC 22%; RIV 20%) and the most common SAE was febrile neutropaenia (RSC 11%; RIV 6%). ARR rates were similar between RSC and RIV pts overall (28%/arm), during cycle 1 (11% vs 14%) and cycles 2–8 (22% vs 20%). From cycle 2–8, the most common ARR by System Organ Class (SOC) was general disorders and administrative site conditions (RSC 10%; RIV 4%). Rates of grade ≥3 ARRs was similar between arms overall (2% vs 5%), during cycle 1 (0% vs 2%) and cycles 2–8 (2% vs 4%); from cycles 2–8, the most common grade ≥3 ARR by SOC was blood and lymphatic system disorders (RSC 1%; RIV 3%). In total, 52% (RSC) and 54% (RIV) of pts had R-related AEs; the most frequent R-related AE was neutropaenia (RSC 14%; RIV 16%). Overall, 8% (RSC) and 9% (RIV) of pts discontinued R due to AEs, most commonly infections (RSC 2%; RIV 3%). In total, 63 pts (11%) died (RSC 10%; RIV 13%). AEs led to death in 6% (RSC) and 7% (RIV) of pts, most commonly due to infections (2%/arm).
Summary
RSC and RIV plus CHOP had similar efficacy and safety outcomes in previously untreated DLBCL. RSC offers convenience for pts and efficient utilisation of healthcare resources.
Keyword(s): CHOP, Diffuse large B cell lymphoma, Rituximab

Session topic: Optimization and innovation in treating aggressive lymphomas
{{ help_message }}
{{filter}}


